Scatterings
A Fleeting Look at the Details of Photoemission
Thanks to cutting-edge ultrafast lasers, an international research team has measured how long it takes an atom to emit an electron after it has absorbed a single photon.
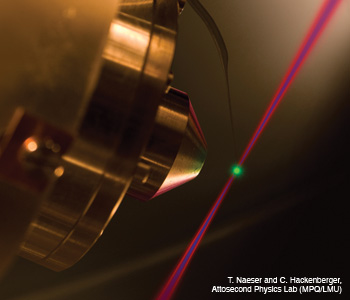 Photoemission of electrons by an attosecond light pulse (blue beam) is time-resolved by controlling the electron motion with an ultrashort visible laser pulse (red beam). This attosecond streaking reveals that electrons from different atomic orbitals are released with a delay comparable to the atomic unit of time.
Photoemission of electrons by an attosecond light pulse (blue beam) is time-resolved by controlling the electron motion with an ultrashort visible laser pulse (red beam). This attosecond streaking reveals that electrons from different atomic orbitals are released with a delay comparable to the atomic unit of time.
Physicists have known about the photoelectric effect for well over a century, but some of the details have remained shrouded in mystery. For instance, after an atom has absorbed a single photon, how long does it take for it to emit an electron? And does that tiny lag time depend on which orbital expels the electron?
…Log in or become a member to view the full text of this article.
This article may be available for purchase via the search at Optica Publishing Group.
Optica Members get the full text of Optics & Photonics News, plus a variety of other member benefits.
