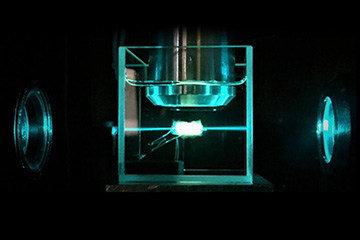
Tumor in the ultramicroscope, illuminated by the light sheet. [Image: TU Wien]
“We got all of it.” Those are the words every cancer patient wants to hear following surgery to remove malignant tumors. But available histopathology methods to confirm the removal of cancerous tissue have limitations, and doctors sometimes are not sure if they really did “get it all.” In those cases, to prevent cancer recurrence, patients are often wheeled back into surgery or sent for additional therapy like radiation.
Now, researchers in Austria and Germany say that a combination of chemistry and light-sheet ultramicroscopy may deliver 3D images of resected malignant tumors in unprecedented detail—a process they demonstrated in experiments on human breast tissue (Sci. Rep. doi: 10.1038/s41598-020-71737-w).
Imaging with ultramicroscopy
“In principle, we will be able to scan every cell of the tumor,” says research team leader Hans-Ulrich Dodt, professor of bioelectronics at Technical University of Vienna, Austria. “That is what is revolutionary about our method.”
Although not yet available in the clinic, Dodt says, the method can be performed in a practical post-surgical time period of one to three days. And the team hopes to make it even faster—perhaps a matter of hours.
Dodt says the work on tumors follows studies of mouse brain and spinal cord imaging with ultramicroscopy he pioneered 15 years ago. In that work, he and colleagues “cleared,” or made transparent, mouse brains using organic solvents and were then able to study the structures with laser-based ultramicroscopy.
In ultramicroscopy, 488-nm light from a diode laser illuminates the study tissue from both sides by two co-localized thin sheets of light. This induces fluorescent signals in a horizontal layer of the tissue, which are picked up by the microscope objective. The sheets of light penetrating the tissue from opposing sides compensate for the absorption gradient within the tissue.
“You can make big stacks of tissue [images] like with computed tomography but it’s much higher resolution,” Dodt says.
Getting to clear
Dodt said he and other researchers realized that any biological tissue can be studied with the ultramicroscopy method if it can be made transparent. The Austrian–German team chose to study breast cancer tissue in part because breast cancer surgeries often result in insufficient “seams” of noncancerous host tissue surrounding the excised tumor, as determined by pathologists using conventional methods.
For decades, he says, oncologists have relied on pathologists’ workhorse 2D histopathology method, in which excised tumors are first preserved in formalin, embedded in paraffin wax and then cut into thin slices for mounting on microscope slides, staining and further study. But even these thin slices can miss cancer cells hiding between sliced layers.
Reversible dehydration
For the breast cancer study, Dodt wanted to find a faster, nondestructive method of “clearing” tissue than was used in the mouse brain studies. The team came upon a chemical dehydration process that rapidly drives water from tissues. First, they fix and enhance tissue autofluorescence with formalin5-/sulfosalicylic acid followed by ultrafast dehydration with 2,2-dimethoxypropane and then refractive index matching with dibenzyl ether.
“This goes much, much faster [than previous methods],” Dodt says, taking from just a few hours to one day. “You can get the tumor very quickly transparent.”
Importantly, the dehydration clearing method is also completely reversible. According to Dodt, in this way cancerous tissue could be studied with light-sheet ultramicroscopy and then rehydrated for 2D histopathology, including tissue and immunohistochemistry staining.
“Pathologists always want to check the results of the new method with what they are used to seeing,” Dodt says of the reversible process. “It works perfectly. Nothing has changed [in the cleared tissue].”
Toward routine, optical postop pathology
Dodt says the clearing and ultramicroscopy method could be used to study excised tumors of many types of cancer. Looking ahead, he hopes to improve the speed of the process as well as work out specific staining protocols, including antibody stains to further improve the ultramicroscopy images.
“Due to its inexpensive, fast and easy performance [the 3D histopathology method] should be applicable for routine postoperative pathology in a not-so-far future,” the team writes.
Ultramicroscopy would also provide the pathologist with additional information about the 3D structure of the tumor, which could be helpful for its classification, Dodt says. Moreover, he adds, as ultramicroscopy produces many thousands of optical sections, the method would be ideal for the application of artificial intelligence (AI) programs to the big data volumes created. This may in the future lead to some kind of computer prescreening of tumor histology, relieving pathologists from some routine work, he says.
