
Christine Hendon and first author Rajinder Singh-Moon, a postdoc at Columbia University, USA, in the lab. [Image: John Abbott]
People with ventricular tachycardia—a type of arrhythmia caused by sudden electrical storms in the heart—face a high risk of sudden death without medical intervention.
Now, a near-infrared spectroscopy (NIRS) method under development by a multidisciplinary team at Columbia University, USA, might one day be used to image and guide more-effective treatment of the disorder (Biomed. Opt. Exp., doi 10.1364/BOE.394294).
Errant electrical activity
At its root, “v. tach.,” as the condition is more colloquially known, is caused by errant electrical activity in the heart’s ventricles. These errant signals can result in a too-rapid heartbeat that doesn’t pump enough blood to the rest of the body and, ultimately, cardiac arrest, says co-author Christine P. Hendon, an electrical engineer and principal investigator of Columbia’s Structure Function Imaging Laboratory.
A treatment, radio frequency (RF) ablation, Hendon explains, can apply heat energy to destroy source points of problematic electrical activity in the heart muscle. Conventional RF ablation approaches, however, may fail to reach v. tach. problem points on the exterior (or epicardial) surface of the heart. As a result, these ablations are unsuccessful in about one third of v. tach. patients, she says. Epicardial RF ablation is a promising solution but still faces challenges that limit accurate guidance and placement of the ablation lesions.
Generating optical maps
“If we can generate optical maps to guide the RF ablation procedure, we can improve success rates and decrease the number of procedures a patient might need as well as length of the procedure,” says Hendon. Other team members include pathologist Charles C. Marboe and cardiologist Elaine Y. Wan, both of Columbia University’s College of Physicians and Surgeons.
Existing therapies for v. tach. include medication and implantable defibrillators that shock the heart back to normal rhythms. But, for some patients, treatment with RF ablation is necessary to treat and potentially cure the patient of their arrhythmia, Hendon says.
For the procedure, a catheter is first used to create an electro-anatomical map of the heart to identify areas of low voltage that are typically due to the presence of scar tissue in the heart. When doctors identify source-points of electrical malfunction, these can be destroyed by creating lesions with RF energy.
Therapy drawbacks
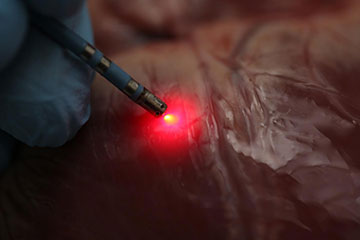
An ablation catheter incorporating near-infrared spectroscopy mapping can successfully distinguish various tissue types in hearts. [Image: Christine P. Hendon, Columbia University. Photographer: John Abbott]
But there are drawbacks to this approach, Hendon says. First, functional mapping alone cannot always distinguish between fat and scar tissue, and RF lesions may be difficult to place and confirm placement, or they may be misplaced. What’s more, the technique can misidentify critical structures like vessels on the outer surface of the heart, leading to surgical complications if ablated.
Using CT or magnetic resonance imaging to guide the procedure, Hendon says, can pose radiation hazards for medical staff or add prohibitive costs.
With NIRS, Hendon says, doctors would receive information about the tissue structure and composition from the optical signals that can be used in conjunction with functional measures from electro-anatomical mapping. The future goal is to have these two functionalities in the same catheter device.
“We think our technology will be cost effective, getting optical signals from living tissue in real time with high sensitivity and a high frame rate,” Hendon says. She notes that light from a NIRS system would have no effect on living tissue and, in fact, NIRS is used in other biomedical applications such as breast and brain imaging.
Guiding light
For the study, the team used human hearts from deceased patients. The donor hearts were ablated, then 3D scanned to obtain a point cloud of their surface geometry. Optical measurements recorded absorption spectra of fat, which were used to create an optical-index algorithm. Afterwards, they confirmed accuracy of NIRS mapping with histopathological examination of the hearts.
For the measuring instrument, the team fabricated a custom aluminum-tip electrode to hold optical fibers for NIRS illumination and collection. The system was integrated with a commercial ablation catheter. The distance between the illumination and collection fibers was adjusted to favor measurement of tissue absorption of light and minimize the influence of light scattering. They used a broadband lamp to illuminate tissue and collected light was recorded with a spectrometer.
The end goal, Hendon says, is development of a new device compatible with current systems for RF ablation. Next steps will include NIRS mapping studies within living animal models with arrhythmia, she says.
