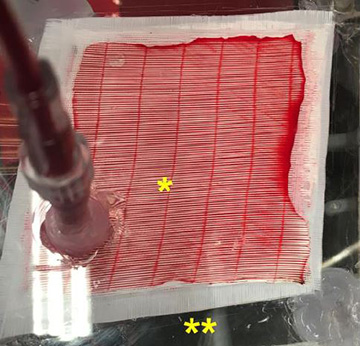
The carbon monoxide photo-remover consists of sealed compartments for blood (marked with *) and gas (marked with **). [Image: L. Zazzeron et al., Sci. Transl. Med. (2019)]
Carbon monoxide kills silently because the odorless, colorless gas deprives the blood’s hemoglobin of oxygen. Administering pure oxygen to poisoned patients may save their lives, but the treatment is more likely to fail if their lungs are severely injured.
Now researchers at a U.S. hospital have devised a light-based method of getting oxygen into the bloodstream without involving the lungs (Sci. Transl. Med., doi: 10.1126/scitranslmed.aau4217). The scientists at Massachusetts General Hospital (MGH) shone light onto blood passing through a device called a membrane oxygenator, where visible wavelengths of light break the bonds holding CO and hemoglobin molecules together.
An old idea, revisited
The Scottish physiologist J.S. Haldane discovered visible light’s ability to dissociate CO from hemoglobin way back in 1896. The idea, however, languished for decades—until the hospital’s photomedicine laboratory picked up on it.
The MGH researchers built their membrane oxygenator from eight stacked layers of microporous polypropylene membrane (taken from a heart-lung bypass machine), a clear plexiglass case with ports for blood inflow and outflow, and 750-mW LED lamps at three wavelengths: blue (460 nm), green (523 nm) and red (623 nm). The device was also ventilated with 100 percent oxygen.
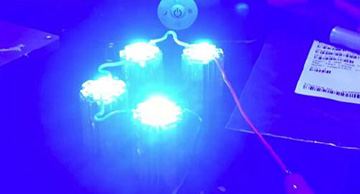
TThe researchers used custom-made light sources such as blue LEDs in their phototherapy. [Image: L. Zazzeron et al., Sci. Transl. Med. (2019)]
Adept CO remover
After basic calibration of oxygenating performance of the device, which the team called a “CO photo-remover,” the scientists filled it with circulating human blood that had already been removed from the body and treated with excess carbon monoxide. A series of experimental runs showed that exposure to the combination of light and oxygen removed more carbon monoxide from the blood than oxygen alone, and that red light was more effective than green or blue wavelengths.
Next, the MGH researchers tested the device on the blood of live rats that had been poisoned by breathing a high concentration of carbon monoxide for 30 minutes. Much like a surgical bypass machine, the equipment diverted some of the rats’ blood through the photo-remover and back into their bodies. Again, arterial blood concentrations of CO decreased faster via red-light phototherapy than by control methods.
Simulating real-world perils—in rats
Finally, to simulate lung damage such as from smoke inhalation during a house fire, the team exposed the anesthetized rats to even more carbon monoxide, until they suffered from tissue hypoxia and lactic acidosis, and gave the rats less-oxygenated air to breathe during treatment. The addition of red-light therapy to the blood resulted in better oxygen levels and reduction of metabolic problems in the rats. (In other words, the control rats, which had lung injury and CO poisoning but were treated only with oxygen, died, while the rats treated with the red-light photo-remover survived.)
So far, the MGH team has fully tested the phototherapy method only on rats, and the researchers say that a bigger version of the device must be tested on larger animals before human clinical trials can begin. A photo-remover capacious enough to treat an adult human—at the same red-light irradiance of 80 mW/cm2—might require 150 to 250 W of electrical power, which would be a consideration in a unit designed to treat patients in the field before they reach the hospital.
