![]()
MIT postdoc Grace Han was part of a team that developed a light-activated chemical compound that can function as a kind of thermal battery. [Image: Melanie Gonick/MIT]
We’re all intimately familiar with batteries that chemically store electrical energy, to be drawn on to run everything from smartphones to the new Tesla semi. A team of scientists at the Massachusetts Institute of Technology (MIT), USA, now has hit upon a way to create a “battery” for storing and releasing heat on command—at the flip of a light switch (Nat. Commun., doi: 10.1038/s41467-017-01608-y).
The system works by collecting solar heat or waste heat from the environment, and using it to melt an organic phase-change material that stores up latent heat as it melts. The twist is that the material in this case is doped with a photoswitching compound that, when treated with UV light, changes the subtle molecular structure of the material in a way that prevents it from solidifying again and releasing its stored latent heat as the material cools. Exposing the material to visible light reverses the photoswitch, allowing the material to solidify and release its latent heat.
The researchers believe that the approach, when perfected, could offer an interesting alternative for using solar energy to power cooking and heating after dark—especially in low-resource areas.
Finding the right energy barrier
Phase-change materials are an intuitively simple concept. The wax of a candle, for example, melts into a pool as it draws in latent heat from the flame, and then solidifies, releasing the briefly stored latent heat, when the flame is blown out and the liquid wax cools. And therein lies the problem: while such materials do a great job at storing and releasing latent heat, the temperature at which they do so is fixed, and the process essentially uncontrolled.
Seeking a path toward longer-term heat storage, the MIT team—led by Jeffrey Grossman, and including postdoctoral researchers Grace Han and Huashan Li—focused on the problem of coming up with an energy barrier to block the liquid-solid transition. Such a barrier could keep a phase-change material in a liquid state well below the temperature at which it usually solidifies, allowing it to store latent heat at lower temperatures.
All well and good—but how could they control the release of that heat for an actual application, in the way that a battery allows controlled release of electrical energy? The answer turned out to rest in light.
Photoswitchable dopant
The scientists reasoned that, if they could impregnate the material with a light-activated, or photoswitchable, compound, they might be able to use light to control the structure of the phase-change material—and, thus, the detailed intramolecular interactions that govern the liquid-solid transition. In essence, as the name implies, the photoswitchable dopant would act as an intrinsic light switch, dialing down the solidification temperature of the material for long-term storage of latent heat. Then, when it was time to harvest the stored heat, another dose of light would reverse the change, allowing the material to solidify and the latent heat to be released.
To test out the concept, the team began with a conventional organic phase-change material, tridecanoic acid, a fatty acid with a high latent heat of fusion. The researchers then doped the material with another organic compound, azobenzene, a chemical photoswitch that undergoes well-understood changes on treatment with light. In particular, when hit with UV radiation at a specific wavelength, azobenzene molecules change from a planar to a twisted isomer; illumination with visible light reverses the isomerization, taking the molecule back to planar form.
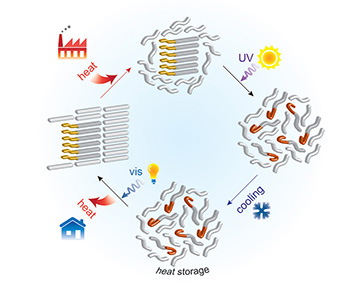
In the MIT team’s system, a phase-change material is treated with a photoswitchable dopant that, when exposed to UV light, changes into a structure that disrupts the intramolecular interactions of the surrounding material, allowing it to stay in a liquid phase well below its usual melting point. Reversing the structural change with visible light allows the material to solidify again and release its stored latent heat on command. [Image: Courtesy of the researchers]
Flipping the light switch
The team then exposed a mass of the doped phase-change material to heat, allowing it to absorb the thermal energy and partially melt, and used a 365-nm UV lamp to illuminate the material—and to kick off the azobenzene isomer change. The change in the structure of the dopant molecules, in turn, disrupted the packing of the molecules of the surrounding phase-change material, preventing it from resolidifying at its usual crystallization temperature. As a result, the researchers found, the material remained in the liquid phase, and holding onto its stored latent heat, some 9 °C below its usual liquid-to-solid transition temperature.
Then, the researchers used a blue LED lamp to illuminate the melted material. The blue LED caused the azobenzene molecules to snap back into their planar form. With the dopant energy barrier switched off, the molecules of the phase-change material quickly began to recrystallize, and to release the latent heat that the material had stored when it melted. Result: A light-switchable heat battery.
A box of stored heat
The team still has many kinks to work out in getting to a production-ready system. Still, the scientists see wide potential for the technique they’ve described. In a press release, Grossman characterized the addition of a light-activated molecule as “a new kind of control knob” for phase-change properties of materials that could have a range of applications.
One such application, as envisioned by the team, might be a portable heat source for uses such as cooking or nighttime heating in low-resource areas. Such a device might consist of a simple container of the material, with covers or slats to control light absorption and allow the material to be stored in darkness. The unit could store up thermal energy from the sun during the day, then be charged with UV light to lower its solidification temperature, and stored in the dark until the heat was needed. At that point, opening the covers on the box would expose the material to visible light, triggering the reverse isomerization and allowing the stored heat to be used.
While solar-heat storage is perhaps easiest to envision, Han stresses that the system can work, in principle, to recycle heat from any source. “The availability of waste heat is widespread, from industrial processes, to solar heat, and even the heat coming out of vehicles,” she says, “and it’s usually just wasted.” She suggests that the team’s light-triggered system for harvesting latent heat could offer a way to put some of that wasted thermal radiation back to productive use.
