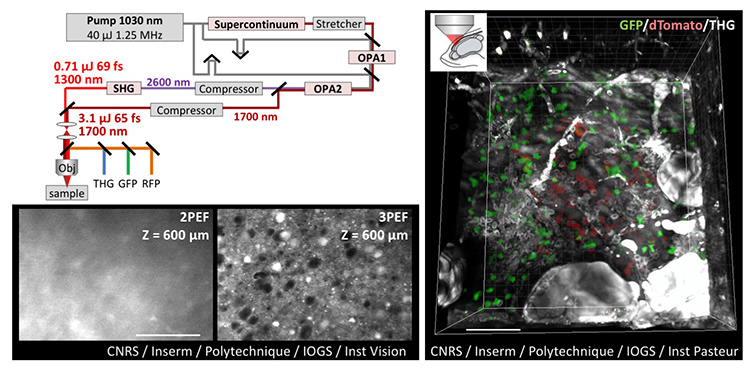
 Top left: Design of dual-color femtosecond OPA providing simultaneous excitation at 1.3 µm and 1.7 µm. Bottom left: Comparison of two- and three-photon deep imaging in fixed mouse brain. Right: THG/3P in vivo imaging of adult zebrafish brain labeled with green and red fluorescent proteins. Scale bars: 100 µm. [© CNRS, Inserm, École polytechnique, IOGS, Institut de la Vision, Institut Pasteur / Springer-Nature. Adapted from reference 2.] [Enlarge figure]
Top left: Design of dual-color femtosecond OPA providing simultaneous excitation at 1.3 µm and 1.7 µm. Bottom left: Comparison of two- and three-photon deep imaging in fixed mouse brain. Right: THG/3P in vivo imaging of adult zebrafish brain labeled with green and red fluorescent proteins. Scale bars: 100 µm. [© CNRS, Inserm, École polytechnique, IOGS, Institut de la Vision, Institut Pasteur / Springer-Nature. Adapted from reference 2.] [Enlarge figure]
In-depth imaging of intact biological tissues, with subcellular resolution and molecular specificity, is a key enabling technology in neuroscience and other fields of biology. Two-photon (2P) microscopy, the reference technique for tissue studies, delivers 3-D fluorescence at depths of few hundreds of micrometers inside scattering environments such as the brain. The strong performance of 2P stems from the robust confinement of multiphoton excitation and the use of near-infrared excitation in the 0.7-to-1.2-µm range. Reaching larger depths, however, is hampered by light scattering that ultimately degrades excitation confinement, resulting in a severely reduced signal-to-noise ratio at depths of a few scattering lengths.
A recently introduced and promising approach for deeper fluorescence microscopy relies on the use of three-photon (3P) excitation in the short-wavelength infrared (SWIR) range, near 1.3 µm or 1.7 µm. The 3P excitation provides better confinement than 2P excitation, and these two SWIR windows offer a good compromise between reduced scattering and minimal water absorption.1 Yet 3P imaging has thus far been restricted to monochrome imaging, because of the large spectral gap between the wavelength windows of interest. Given that many applications require visualizing interactions between cells or tissue components labeled with different fluorophores, multicolor laser sources enabling efficient two-color 3P imaging constitute an important research target.
To meet this challenge, we developed a novel source emitting concomitant femtosecond pulses at 1.3 µm and 1.7 µm.2 We showed that the 1.7-µm pulse provides efficient 3P excitation of red fluorophores (RFPs), while the 1.3-µm pulse targets the green fluorescent protein GFP.
Our strategy was to design a MHz-rate optical parametric amplifier (OPA) providing µJ-range pulses at 1.7 µm, and to frequency-double the 2.6 µm idler beam to obtain a second output at 1.3 µm. This scheme provided unmatched performance, optimized for multichannel 3P microscopy (less than 70 fs, 1.25 MHz and µJ pulse energy in each beam).
The laser outputs were then routed onto a custom-built multiphoton microscope, optimized to work in this excitation wavelength range. GFP, RFP and third-harmonic generation (THG) signals were picked up on three independent detectors. We could then record dual-color multimodal three-photon 3-D images of both fixed and live neural tissues.2 In particular, we obtained the first in-depth images of live adult zebrafish brains with two distinctively labeled cell populations. This work should open many perspectives in neuroscience, developmental biology and biomedical sciences.
Researchers
L. Abdeladim, P. Mahou, W. Supatto and E. Beaurepaire, École Polytechnique, Palaiseau, France
K. Guesmi, K. Jurkus, P. Rigaud, P. Georges, M. Hanna and F. Druon, Institut d’Optique–Graduate School, Palaiseau, France
S. Tozer, T. Kumamoto, K. Loulier and J. Livet, Institut de la Vision, Paris, France
N. Dray, Institut Pasteur, Paris, France
References
1. N.G. Horton et al. Nat. Photon. 7, 205 (2013).
2. K. Guesmi et al. Light Sci. Appl. 7, 12 (2018).
