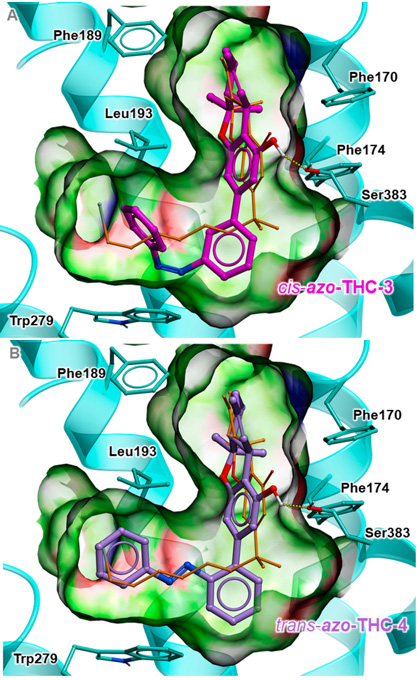
Predicted binding poses for active cis-azo-THC-3, shown as sticks with magenta carbon atoms (A); and for active trans-azo-THC-4, shown as sticks with purple carbon atoms (B). [Reprinted from M.V. Westphal et al., J. Am. Chemical Soc. 139, 18206 (2017), with the permission of ACS Publications.]
Tetrahydrocannabinol (THC), the psychoactive ingredient in marijuana, has been enjoyed recreationally for ages. However, the intoxicating molecule is also of interest to researchers because components of the endocannabinoid system—THC and its cell-membrane receptors—could be starting points for new treatments for pain, addiction, obesity, depression, and neurological diseases like Alzheimer’s and Parkinson’s.
Now, researchers from the Swiss Federal Institute of Technology (ETH) in Zurich have created photosensitive forms of the THC molecule that they say will help them to better understand what happens when THC binds to cannabinoid receptors in the cell membrane (J. Am. Chem. Soc., doi: 10.1021/jacs.7b06456).
Cannabinoid receptors and fat-loving THC
There are two types of cannabinoid receptors, conveniently named cannabinoid receptor one and two (CB1, CB2). The ETH team focused its study on CB1 receptors, which are mostly found in the central nervous system and have been linked to mood, motor coordination, memory and cognition. (CB2 receptors are more common in the peripheral nervous system and are linked to the immune system and pain response.)
Understanding the diverse interactions between THC and CB1 would help researchers learn more about the role CB1 plays in cell-signal transduction and disease; however, THC is hard to study because it is a lipophilic (fat-loving) molecule, and its shape sort of melts when it docks to CB1 in the cell membrane’s lipid bilayer. To make studying the interaction between THC and CB1 easier, the ETH team—led by Erick Carreira—created four synthetic THC molecules whose shape can be altered with light.
These designer THC molecules have light-sensitive photoswitchable azobenzene (a commonly used chromophore) tags and can be optically manipulated using specific wavelengths of light. For example, exposing the azo-THC molecule to ultraviolet (UV) light changes its spatial structure, and exposing the same molecule to blue light will reverse the effect.
azo-THC and CB1 binding in living cells
The researchers found that azo-THC-3 and azo-THC-4 were the most stable and robust photoswitchable ligands of the four molecules they created. They used these two molecules in live cell cultures in proof-of-principle experiments to show that their “light-sensitive THC variants are a suitable tool for controlling and influencing CB1 receptors.”
After confirming that the azo-THC molecules bound normally with CB1 receptors in the membranes of the live cells, the research team exposed the azo-THC molecules to UV light (365 nm). The molecules’ spatial structure changed as the researchers predicted, which activated CB1 and caused potassium ion channels in the membrane to open. The potassium ion flow was measured by an electrode the researchers had inserted into the cell culture prior to the experiment.
When the same azo-THC molecules were exposed to blue light (450 nm), the molecules’ spatial structure changed to its original form, which, in turn, deactivated the CB1 receptor. Again, this was confirmed by the electrode measuring a halt in potassium ion flow. The researchers were able to repeatedly activate and deactivate the CB1 receptor without introducing new azo-THC molecules.
Other members of the ETH team are working on testing additional optically controlled THC molecules—these are tagged with a photoswitch that responds to long-wavelength red light, which can penetrate deeper into human tissue than blue light.
