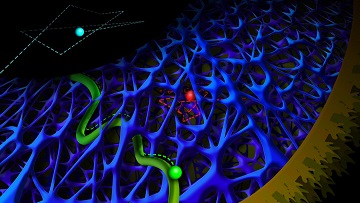
Light-emitting quantum dots trace rapid diffusion (cyan) and slow diffusion (red) in an intracellular actin network, and active transport by motor proteins (green) inside a cell. [Image: Anna Vinokurova]
Researchers from Utrecht University, Netherlands, report delivering quantum dots (QDs) into a cell and using them to view (via rapid live imaging) how the cytoskeleton affects intracellular dynamics (Nat. Commun., doi: 10.1038/ncomms14772). The team, led by Lukas Kapitein, says that its work could “increase our understanding of the material properties of the cytoplasm, guide future modeling approaches, and could potentially aid studies of passive delivery of nanoparticles or therapeutic agents.”
The cytoskeleton serves a purpose similar to scaffolding, and is made up of actin, intermediate filaments and microtubules that support the cell’s shape and act as a highway system for kinesin motor proteins transporting large molecules. Small molecules, however, can diffuse through the cytoplasm—unaided—through holes in the cytoskeleton.
Transport via diffusion
How these transport mechanisms are affected by the organization and dynamics of the cytoskeleton is unclear. At only a few nanometers wide, QDs are small enough and bright enough, according to the researchers, to investigate these “poorly understood” intracellular dynamics up close and in live cells.
The Utrecht scientists used QDs in the role of small molecules to observe diffusion in action. They used adherent cell electroporation to move the QDs across the cell membrane and into the cytoplasm without having to first encase the QDs in movement-inhibiting vesicles. Once inside the cell, the researchers recorded the diffusion speed of the QDs through the cytoplasm.
The researchers found that the QDs had slower diffusion speeds in areas close to the cell membrane, since those areas had closer-knit filamentous actin (F-actin) in the cytoskeleton than the areas deeper in the cytoplasm. (Imagine running through a heavily wooded forest versus running through an open field.) They confirmed actin’s role in slowing down the QDs by observing faster QD-diffusion speeds in cells that were treated with an inhibitor to break apart F-actin.
Notably, these faster diffusion speeds were only detectable because the QDs didn’t cause photobleaching, and because they were sufficiently bright to let the researchers film the dots’ movements at 400 frames per second—10 times faster than normal video.
Transport via kinesin motors
Kinesin motor proteins “drive” their cargo—molecules or organelles that are too big to move via diffusion—on the microtubule highways of the cytoskeleton. These microtubule highways can bend, but the mechanism that controls this fluctuation remains unclear. In hopes of shedding light on this mystery, the researchers tagged kinesin motor proteins with QDs and observed their intracellular travels.
Of the four kinesins they observed, only kinesin-1 showed subtle directional fluctuations in trajectory. These results contradict other reports of motor-driven particles undergoing large sideways fluctuations in their trajectories. And when they applied F-actin polymerizing and depolymerizing agents to the cytoskeleton, they saw that depolymerization suppresses microtubule shape remodeling. Therefore, the authors say, microtubule fluctuations are not, as previously proposed, caused by a contracting F-actin network, but are instead suppressed by it.
