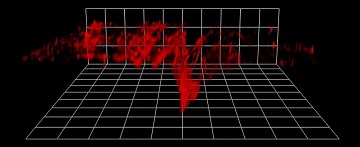
3-D two-photon excitation microscopy of microdamaged bovine bone. [Image: Esther M. Surender, Trinity College Dublin, Ireland]
Scientists from Ireland have developed and demonstrated a biologically safe, luminescent nanoagent for imaging tiny microcracks in bone (Chem, doi: 10.1016/j.chempr.2016.08.011). The nanoagent selectively binds to cracks in the bone’s surface, and lanthanide complexes tethered to the nanoagent fluoresce to highlight these damaged areas.
The researchers were able to capture and combine fluorescence patterns to create 3-D maps showing the location and depth of microcracks in bone samples. They say that their nanoagent could be part of an early-warning system for skeletal diseases like osteoporosis.
In vitro demonstration
Everyday activities can cause tiny cracks in our bones. If our bodies can’t keep up with repairing this damage, the cracks multiply, resulting in stress fractures and degenerative bone diseases. Currently, detection of bone microcracks is done using colorimetric dyes—which cannot distinguish between new damage and repaired bone—and X-rays, which have been associated with an increased risk of cancer.
The Irish research team, led by Thorfinnur Gunnlaugsson and Esther M. Surender of Trinity College Dublin (TCBI), has now come up with new lanthanide-based surface-modified gold nanoparticles, ideal for imaging microdamage in bones and not requiring X-rays for detection. To test the system out, the researchers scored polished bone samples with a scalpel to simulate microcracks. Next, they incubated the samples in a solution containing the nanoagent. After incubation, the researchers employed a sensitizing antennae to activate the nanoagent’s fluorescence, and two-photon excitation (TPE) microscopy to record the signal.
Results from the demonstration showed that the fluorescence signal was concentrated at the microcracks and not the smooth areas of the bone, confirming the nanoagent’s ability to selectively bind to the calcium ions that are exposed when bone is damaged.
The researchers captured z-stack fluorescence plots of the scored bone samples—from the outer surface of the bone to the bottom of the microcracks—and combined them to generate 3-D maps of the damaged areas.
Biologically safe
Authors of the Chem paper say the nanoagent has features that make it attractive for use in vivo. For example, gold nanoparticles are already commonly used in clinical applications, including drug delivery, sensing and imaging. And the specificity and small size of the nanoagent means a lower concentration is needed for imaging.
Other members of the research team include Steve Comby, TCBI; and Brenton Cavanaugh, Orlaith Brennan, and Clive Lee, Royal College of Surgeons in Ireland.
