Adding a third objective lens allowed researchers to capture previously neglected fluorescence, improving image resolution in three dimensions for techniques such as light-sheet microscopy. [Image: Yicong Wu, National Institutes of Health, USA]
A research team from the United States and China has demonstrated a three-lens fluorescence microscopy method, paired with a computational algorithm, that uses “lost” light from biological specimens to produce high-resolution 3-D images (Optica, doi: 10.1364/OPTICA.3.000897). The scientists say that their new method, called tri-view plane illumination microscopy (triSPIM), more than doubles volumetric resolution compared with traditional fluorescence techniques—without “compromising acquisition speed or introducing additional phototoxicity.”
Fluorescence microscopy is commonly used in biological imaging, but conventional setups can’t collect all of the fluorescence emitted by the specimen. The escaped light takes with it information that could be used to create sharper images. With triSPIM—an improvement on the team’s dual-view design—a lens mounted below the specimen captures this lost fluorescence signal at the same time views are taken from two lenses mounted above the specimen. Raw image data from these three views are processed to create a single image with improved spatial resolution.
The scientists, led by Hari Shroff at the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, USA, demonstrated triSPIM using light-sheet and wide-field modes of fluorescence microscopy on a variety of biological specimens:
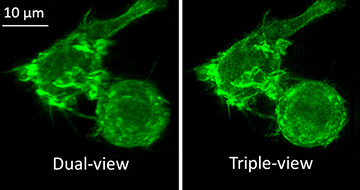
Actin in a white blood cell labeled with green fluorescent protein, imaged with the dual-view microscope (left) and with the new triple-view microscope (right).
[Image: Yicong Wu and Valentin Jaumouillé, National Institutes of Health, USA]
Triple-view light-sheet mode. During a triSPIM light-sheet mode demonstration, the team alternately employed a light sheet from two upper lenses tilted 45 degrees relative to the third lens located below a slide containing live white blood cells. Two axial views were simultaneously captured by sCMOS cameras attached to the upper lenses. The third fluorescence view was captured by a sCMOS camera when the scientists mechanically swept the lower lens’ plane of focus vertically through the cells.
The fluorescence view was computationally fused to the other two views via a joint deconvolution algorithm, developed in collaboration with co-author Patrick J. La Riviére’s group at the University of Chicago, USA. The resulting images showed a 1.4-fold improvement in lateral resolution relative to the team’s dual-view design (see image).
Triple-view wide-field mode. During a wide-field mode demonstration, the scientists illuminated a sample of live Bacillus subtilis bacteria with one of the two upper lenses. All three lenses collected fluorescence views from the bacteria along three detection axes onto sCMOS cameras. After applying the joint deconvolution algorithm to the views, the researchers reported obtaining 3-D images with 340-nm resolution.
The scientists hope to extend the use of their multiview capture-and-fusion method to other microscope modalities and biological applications.
The team worked collaboratively at the Marine Biological Laboratory’s Whitman Center in Woods Hole, Mass., USA.
