Feature
Photoacoustic Tomography and Microscopy
By converting light waves into sound, researchers have developed a high-resolution biological imaging technique that allows them to see living tissue at new depths.
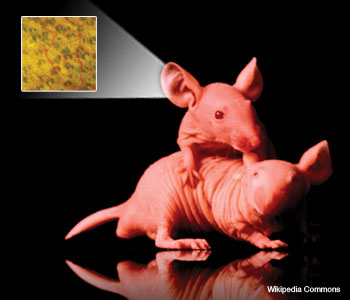 The microvasculature in the ear of a nude mouse is captured using transmission optical microscopy.
The microvasculature in the ear of a nude mouse is captured using transmission optical microscopy.
Biomedical researchers have greatly benefited from commercially available high-resolution 3D optical imaging modalities—including confocal microscopy, two-photon microscopy and optical coherence tomography. Unfortunately, however, such tools cannot penetrate biological tissue deeper than about 1 mm. Photoacoustic tomography (PAT), which combines strong optical contrast and high ultrasonic resolution in a single modality, has broken through this fundamental depth limitation.
…Log in or become a member to view the full text of this article.
This article may be available for purchase via the search at Optica Publishing Group.
Optica Members get the full text of Optics & Photonics News, plus a variety of other member benefits.
