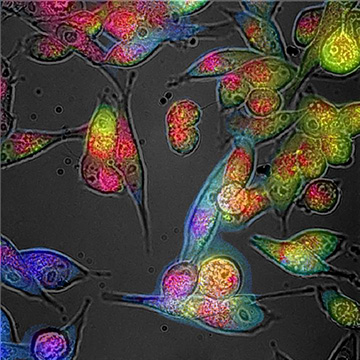
A recently reported technique enables the identification of oxidative stress in cells and tissues via the colors of their autofluorescence response. [Image: Courtesy of Martin Gosnell, Quantitative Pty Ltd.]
Oxidative stress attributable to reactive oxygen species (ROS)—so-called free radicals—can wreak havoc on cells, and is a hallmark of many progressive diseases, certain ophthalmologic conditions, cancer, diabetes and neurodegenerative disorders. Now, a research team led by engineering professor Ewa Goldys at the University of New South Wales (UNSW), Australia, reports an in vivo method that uses wavelengths of light to detect and measure ROS in cells (Redox Biol., doi: 10.1016/j.redox.2020.101561).
Oxidative stress-ed out
“Many diseases are characterized by elevated ROS, in particular some eye diseases,” says Goldys, who is deputy director of USNW’s Center for Nanoscale Biophotonics. While such oxidative stress is not disease-specific, she notes, measuring a return to healthy levels of ROS can constitute one sign of therapeutic success.
“There is a need for noninvasive methods of molecularly sensitive detection of ROS,” Goldys adds. Medical diagnostics has typically overlooked oxidative stress, she says, because it would only be practical to measure it in vivo, and doing such measurements must avoid toxic chemical probes or cell and tissue extraction from deep within the body.
Multispectral imaging
The Goldys team’s technique is based on autofluorescence multispectral imaging (AFMI). The approach starts with an adapted fluorescence microscope that the team has kitted out to have an expanded number of spectral channels, covering specific excitation and emission wavelength ranges. The setup works by sending out bursts of LED light at various wavelengths onto cells and tissues. Fluorescent molecules in the targets absorb the light and emit their own wavelengths of light back in response.
Importantly, the team notes, the technique allows UV wavelengths to be omitted—an especially important capability for in vivo assessment of ophthalmologic or reproductive problems.
“A lot can be done on an adapted fluorescent microscope,” Goldys says, “but simpler hardware based on LEDs and commercial cameras might be used in instances where high magnification is not needed. Many disease conditions do not require microscopy for diagnostics.”
For the study, the team used three different human cell lines plus kidney tissue from mice. “This was a random selection of cells and tissues where we expected to see variable oxidative stress,” Goldys says. “In particular, we used tissue from animals that were exposed to tobacco smoke.”
Fluorophores might signal trouble
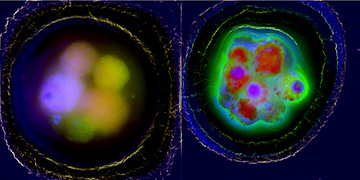
Left: Composite multichannel fluorescence image of healthy bovine embryo cells. Right: Similar image of bovine embryo cells exposed to severe oxidative stress during development. [Image: Courtesy of Martin Gosnell, Quantitative Pty Ltd.]
Fluorophores in cells or tissues include collagen, elastin, tryptophan and reduced nicotinamide adenine dinucleotide phosphate (NADP) and flavins. For some fluorophores, particularly NADP and flavins, autofluorescence depends on oxidative state—a fact that bolstered the team’s belief that ROS could indeed be noninvasively evaluated and monitored.
Previous work by Goldys has shown that the colors of cells and tissues can be indicators of health and disease. Her group has shown how to “unmix” the native fluorescence of cells and tissues into their molecular components by assigning a value to each color component.
In the current study, color values for the AFMI samples were determined by comparison with ROS reference values obtained by traditional in vitro techniques of dye-staining cell and tissue samples. The unmixing to decode ROS values for in vivo diagnostics does require some computing power—“ours is an IT-intensive method,” Goldys says.
Toward broader application
“AFMI provides a promising measurement tool for ROS, which can be translated to future clinical and research applications,” the team writes. Going one step further, Goldys says it is possible the technique could one day be used to determine disease severity and progression based on ROS values.
“We have just scratched the surface of noninvasive medical diagnostics using native colors and shapes,” according to Goldys, who notes that deep-learning and artificial-intelligence methods could become part of the mix.
“We expect we will be able to answer many outstanding biomedical questions, particularly from the areas of neurodegeneration, chronic pain, and reproduction,” Goldys says. The group is, she adds, “actively seeking commercial partners.”
In addition to UNSW scientists, the work included contributions by researchers from the University of Sydney and the firm of Quantitative Pty Ltd., Australia.
