![]()
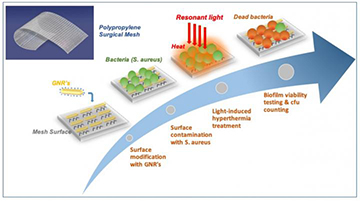
Researchers in Spain impregnated meshes used in a common surgical treatment (mesh pile on right) with gold nanoparticles (mesh pile on left) to create a system that could fight biofilm development on the meshes with infrared light and localized surface plasmon resonances. [Image: ICFO]
Infections by antibiotic-resistant bacteria have emerged as a growing medical threat in recent years. And the problems of fighting such infections intensify when these hardy microbes organize themselves into biofilms—slimy aggregates that protect the bacteria from conventional attack by the body’s immune system. In particular, these nasty films can accumulate on medical devices implanted during routine surgeries, causing post-surgical complications and even death in some cases.
Researchers in Spain have now added a potential weapon to the anti-biofilm arsenal—one that would fight the films using gold nanoparticles and a dose of infrared light (Nano Lett., doi: 10.1021/acs.nanolett.9b00187). While thus far their plasmonic biofilm scourge has been tested out only in the lab flask, the scientists believe it could find use in a range of in vivo settings and applications.
“Cities for microbes”
As the name implies, biofilms—which have been described as “cities for microbes”—are thin aggregates of bacteria that colonize surfaces. The bacteria develop an extracellular polysaccharide matrix (essentially, a binding of sugars, with associated proteins, DNA and fats) that encapsulates the individual bacteria and protects them from the ravages of the immune system, as well as from antibiotic therapies.
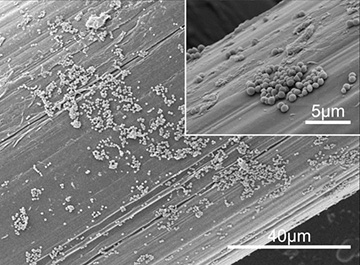
SEM micrographs of Staphylococcus aureus biofilm formed on the surface of a surgical mesh. [Image: ICFO]
Biofilms can do particular mischief when they colonize certain medical devices implanted as part of surgical treatments, such as the synthetic meshes routinely used to repair hernias. Some 20 million hernia repair operations are performed each year—and 1 to 8 percent of those operations are associated with some sort of post-surgical mesh infection. Because the mesh biofilms are so resistant to treatment with antimicrobials, resolving such an infection often requires one or several additional surgical procedures to remove and replace the biofilm-tainted mesh and clean up the infection.
A plasmonic solution
The Spanish research team—which included scientists from ICFO–Institut de Ciències Fotòniques and ICREA–Institució Catalana de Recerca i Estudis Avançats in Barcelona, as well as from the firm B. Braun Surgical, and was led by OSA member Romain Quidant—have now offered a possible solution to the quandary that uses infrared light, plasmonically enhanced.
The team began by developing a chemical technique to firmly anchor millions of gold nanorods, each around 49 nm long and 13 nm in diameter, to the surface of a typical synthetic surgical mesh that would be used in a hernia repair. The size and shape of the nanorods was selected such that they would be subject to a localized surface plasmon resonance (SPR) when struck by light at a near-infrared wavelength of around 810 nm. That wavelength, the researchers note, lies within “the first transparency window for biological tissues (650–1100 nm)”—a wavelength band at which light is less strongly absorbed by tissues, and can thus penetrate deeper.
Breaking up biofilms with heat
In the technique reported by the Spain-based team, gold nanorods (GNRs), attached to the surface of the surgical mesh, are illuminated with infrared light, which leads to a localized surface plasmon resonance in the particles. The localized SPR heats the mesh surface, breaking up any bacterial biofilm that has developed on the surface. [Image: ICFO] [Enlarge image]
In a localized SPR, the size and shape of the nanorods leads to a subwavelength concentration of incident light at the air-metal interface, and stimulates a strong local electron plasma oscillation at the nanoparticle surface. This effect dramatically enhances the local light-field intensity—and gives off a significant amount of heat in the process. To test that effect, the team illuminated the nanorod-infused mesh with an 810-nm continuous-wave laser, and confirmed with an infrared camera that the particles’ localized SPR did indeed heat up the mesh as expected.
After agitating the nanorod-coated mesh in a chemical flask to verify that the nanoparticles would hold fast in a real biological setting, the team next colonized the surface with Staphylococcus aureus bacteria, giving the bacteria time to form a robust biofilm on the nanorod-treated mesh. Then, the researchers treated the biofilm-coated mesh to pulses from a 750-to-1200-nm light source.
The team found that the heat from the localized SPR disrupted and burned off large sections of the biofilms. And even the biofilms that remained had been sufficiently weakened to make the bacteria much more vulnerable to antimicrobial treatments and immune-system cleanup.
Toward better post-surgical outcomes
While the work represents mainly a proof of principle, and was conducted in chemical flasks rather than a real biological system, the team believes that the use of its nanorod-treated meshes could help in the fight against post-surgical complications due to biofilm development.
The researchers point out that—because the approach relies on NIR light in the tissue transparency window—the light to activate the localized SPR and kill the biofilms could be delivered from outside the body if the mesh was less than 1 cm from the surface, and piped in via a small incision for meshes implanted deeper in the body. Either, the team suggests, would be preferable to the current approach of additional major surgery to remove and replace infected meshes. Further, according to the researchers, the temperature increase induced by the nanorods should be sufficiently localized to the mesh to avoid damage to surrounding, healthy tissues.
The next steps for the team will be to further explore the biocompatibility of the nanoparticles and the long-term acceptance of the meshes in animal experiments. Nonetheless, team leader Quidant, in a press release accompanying the work, said that the technique could “signify a radical change in operation procedures and further patient post recovery.”