Near-infrared light triggers the photorelease of caged molecular cargos, allowing for imaging and controlled drug release in a nanotheranostic system. [Image: S. Gerber, EPFL] [Enlarge image]
Even if you’re not familiar with the up-and-coming field of nanotheranostics, the on-the-nose name gives away its goal. The idea is to unite medical “therapeutics” with “diagnostics,” essentially combining drugs or techniques to both diagnose and treat disease—all in a single, tiny package.
Now, a Swiss research team has designed a nanotheraonstic system that combines diagnostic imaging with the light-triggered release of active molecules (ACS Appl. Mater. Interfaces, doi: 10.1021/acsami.9b07954). The researchers believe that their system could be an important precursor to the development of nanocarrier platforms that allow for decoupled imaging and on-demand release of treatments.
Everything’s coming up nano
Nanotechnology is an attractive starting point for designing thernanostic systems, as nanoparticles have great potential both for targeting specific organs or tissues and for delivering a very localized, high concentration of therapeutics, also known as “molecular cargo.” Also, nanoparticles can be designed and manipulated to serve multiple functions, making them ideal vehicles for uniting imaging and treatment applications.
However, nanotheranostics currently faces many roadblocks. Traditional light-activated nanophotonic approaches, such as plasmonic and upconversion nanoparticles, are limited by fixed excitation bands in the high-energy ultraviolet (UV) spectral domain. These wavelengths don’t penetrate living tissue well and can cause photodamage, limiting biomedical applications.
To overcome these limitations, the Ecole Polytechnique Federale De Lausannee (EPFL) and University of Geneva researchers turned to harmonic nanoparticles (HNPs), metal oxide nanocrystals that present a highly efficient nonlinear response, as the nanocarrier for their system. On the imaging side, HNPs have already made a name for themselves as alternatives to fluorescent probes due to their second-harmonic emission in response to excitation by light in wavelengths ranging from the UV to the near-infrared. NIR light provides much deeper tissue penetration and less photodamage than high-energy UV.
Also, HNPs are flexible; they can be tuned to respond to different wavelength activations. This flexibility, the researchers realized, allows for the separation of the imaging functionality from the photo-activation processes that trigger cargo release, and could even enable the sequential release of multiple caged compounds responsive to different wavelengths. HNPs are also highly biocompatible, once coated in silica, and photostable.
Customizing HNPs
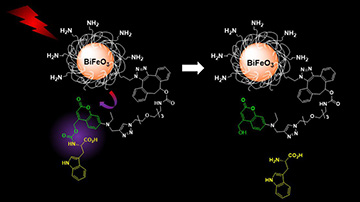
To synthesize the multifunctional nanocarrier system, the team took silica-coated bismuth-ferrite (BFO) HNPs and equipped them with light-activated “caged” molecular cargoes at the nanoparticle surface using a photoresponsive tether. For the purposes of its experiments, the team chose the amino acid L-tryptophan (Trp) for the molecular cargo. (As a key ingredient of the neurotransmitter serotonin, Trp has implications for the treatment of depression.)
The team first tested the photorelease functionality under direct UV irradiation (366 nm). Then, in a two-photon setup, femtosecond-pulsed NIR radiation at 790 nm caused harmonic emission of the HNPs at 395 nm. The second-harmonic emission triggered the severing of the tether, uncaging the cargo and releasing the Trp. Finally, the team monitored and quantified the results using ultra-high-performance liquid chromatography–mass spectrometry, ensuring that the process didn’t induce cytotoxicity in the cells.
According to the researchers, their results demonstrate the first nanocarrier platform based on HNPs, extending the biomedical functionality of these nanoparticles beyond imaging. They also foresee that a wide swath of molecular cargoes, including drugs, could be attached to coated BFO HNPs using this system, and that the system’s tunability could be significant for conjugating several caged cargoes sensitive to distinct excitation wavelengths and controlling the sequential release of different molecules.