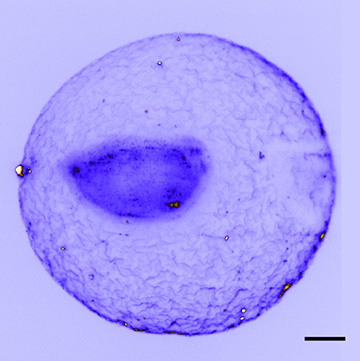
A system for immobilizing live biological specimens for light-sheet fluorescence microscopy allows the details delicate biological specimens, such as the embryo of the amphioxus embryo Branchiostoma lanceolatum, to be captured without damage. (Scale bar: 50 microns) [Image: Z. Yang et al., Nat. Commun., doi: 10.1038/s41467-019-08514-5; image courtesy of University of St. Andrews]
The technique of light-sheet fluorescence microscopy (LSFM) can rapidly provide stunning, 3-D images of intact organs and small organisms, such as zebrafish and mouse brains. But to get those images, the samples usually need to be immobilized in a stiff gel—a demand that can limit the technique’s use in some studies of biological dynamics and in aspects of drug discovery.
Now, a research team led by OSA Fellow Kishan Dholakia of the University of St. Andrews, U.K., has devised a way to marry LSFM with a different technique for holding the specimen in place: a contact-free ultrasound trap (Nat. Commun., doi: 10.1038/s41467-019-08514-5). And the researchers demonstrated the usefulness of the approach by using the LSFM–ultrasound combination to measure the effect of specific drugs on the heartbeat of zebrafish larvae suspended in the trap.
Seeking contact-free immobilization
LSFM works by using optical elements to shape an excitation laser into a thin sheet, which in turn excites fluorescence only in a micrometers-thick slice of the sample. The fluorescence signal from that thin sample slice is then collected in a microscope objective and shunted to a CCD or other image sensor. By taking multiple sections from different angles and orientations, a high-resolution, 3-D image of the specimen can be rapidly built up. In addition to its speed and resolution, LSFM also boasts relatively low photobleaching and photodamage of delicate biological specimens.
But LSFM does have one disadvantage: getting the multiple orientations and sections to build up the 3-D image requires the chamber containing the sample to be moved and rotated, to position it with respect to the thin light sheet from the excitation laser. That requires a way to hold the sample in place within the sample chamber as the chamber moves from position to position.
Commonly, that immobilization is accomplished by burying the sample in a clear agarose gel within the sample chamber—not exactly the natural habitat of model organisms, such as zebrafish embryos, that might be imaged using the technique. Encasing live organisms in the gel can thus disrupt the very biological processes being studied. And for some experiments, such as measuring the metabolic effects of drug candidates, the gel can drastically slow the delivery rate of the drugs to the specimen, making it difficult to gauge their real-time impact.
An acoustic trap
In principle, optical traps or tweezers would offer one contact-free way to hold the specimen in place for LSFM. But while such traps have been used to manipulate objects such as the tiny, 50-micron-diameter ear stones, or otoliths, within zebrafish larvae, the piconewton forces of these traps aren’t sufficient to hold bigger objects such as a whole zebrafish. And the concentrated laser power in optical traps could fry some delicate specimens.
Dholakia’s team at St. Andrews looked at another, analogous contact-free trapping setup—one using sound waves rather than light to hold the specimen in place.
The setup begins with the addition of a bowl-shaped ultrasound transducer on each of two sides of the sample chamber. Counterpropagating acoustic waves from the two transducers are tuned to form a standing wave, and the gradient forces in the acoustic field (analogous to those in optical tweezers) hold the sample in place. The micronewton forces and acoustic-wave length scales involved are the right size for samples ranging from tens of microns to millimeters in size—and the acoustic power is distributed over a larger area than the power in optical tweezers, limiting damage to delicate biological specimens.
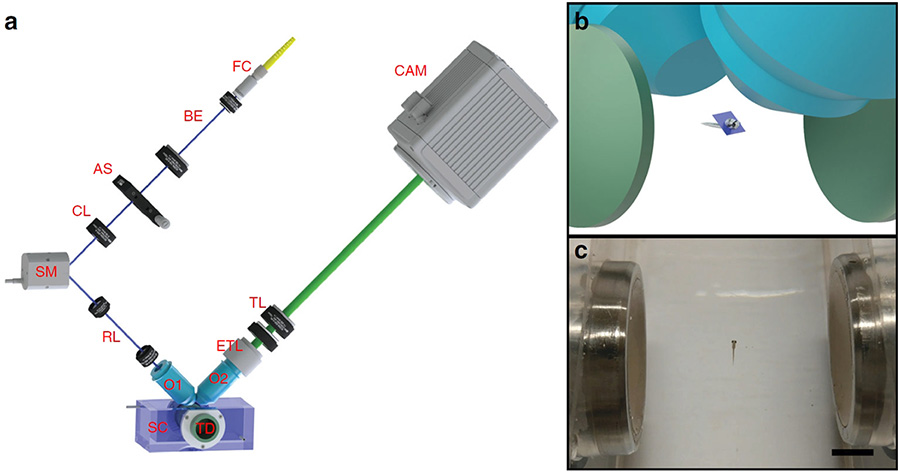
Left: The St. Andrews setup includes acoustic transducers (TD) to hold the sample in place, and a scanning mirror (SM) and electrically tunable lens (ETL) to scan the beam across the sample for light-sheet fluorescence microscopy. Right top: Acoustic transducers (green) set up standing wave to hold sample in place, for imaging through objective lenses (blue). Right bottom: The acoustic trap can immobilize objects as large as a live zebrafish larva. (Scale bar: 5 mm) [Image: Z. Yang et al., Nat. Commun., doi: 10.1038/s41467-019-08514-5; image courtesy of University of St. Andrews]
One challenge for designing the setup lay in the possibility that, in the translation of the sample chamber required for LSFM, the microscope objectives could interfere with the acoustic field and destroy the trap. To get around this, the team added some clever optical elements: a synchronized scanning mirror to scan the light sheet across the specimen through one lens, and an electrically tunable lens to adjust the focal plane on the detection side. The optical setup allows small samples to be scanned without moving the microscope objective or the specimen, and thus without disturbing the acoustic field holding the specimen in place.
Tests on live organisms
The team used the setup to image a variety of intact marine-animal embryos and larvae, suspended by ultrasound in water (their natural environment). The researchers found that the delicate structures of these specimens, such as the fertilization envelope in marine-invertebrate embryos, held up far better under acoustic positioning than when the samples are embedded and immobilized in agarose gel. Time-sequence imaging allowed the team to visualize changes in the embryos’ cell structures over a period of four hours after fertilization.
In another test, the researchers used ultrasound to suspend whole zebrafish larvae in the sample chamber within E3 medium (a saline solution commonly used in zebrafish studies), and then pumped drugs known to affect the fish’s heart rate into the chamber. They were able to rapidly and repeatedly image the fish’s heart using LSFM and, by using optical flow analysis, document the drug’s effect on the fish’s cardiac cycle.
“This is a new way to perform drug-based studies for cardiovascular disease and developmental biology,” Dholakia said in a press release accompanying the work. “We anticipate this approach can also be used for high-throughput drug discovery, an important topic for future healthcare.”
In addition to researchers at St. Andrews, the work included scientists from the University of Edinburgh and the University of Glasgow, U.K., the University of Western Australia, and Illinois Wesleyan University, USA.
