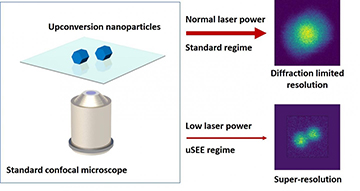
A team of researchers at Macquarie University, Australia, has outlined and tested a system that, using upconversion nanoparticles (UCNPs) as “super-linear” fluorophores, could achieve super-resolution imaging at low laser powers via a conventional confocal-microscope setup. [Image: ARC Center of Excellence for Nanoscale BioPhotonics] [Enlarge image]
Super-resolution microscopy, the Nobel Prize–winning innovation that allows imaging below the diffraction limit of light, has enabled some breathtakingly sharp views of tiny biological structures. But it’s not something you’ll find in a typical biology lab—instead, it requires sometimes complex and costly tools and substantial image post-processing.
Now, researchers at the Macquarie University ARC Center of Excellence for Nanoscale BioPhotonics (CNBP), Australia, have developed a scheme that they say could bring 3-D sub-diffraction imaging capability to the standard confocal microscopes that are ubiquitous in bio labs (Nat. Commun., doi: 10.1038/s41467-019-11603-0). The technique—which the team has dubbed upconversion super-linear excitation–emission (uSEE) microscopy—rests on a clever use, as fluorescent markers, of so-called upconversion nanoparticles (UCNPs), which provide sharp, tunable narrow emissions when excited by low-power, near-infrared light.
Super-linear emitters
The idea of achieving super-resolution on confocal microscopes through the use of “super-linear” emitters actually goes back several decades. In essence, these luminescent markers constitute a class of materials for which fluorescent emission depends super-linearly on the power of the exciting laser.
What that means in practice is that, when a relatively low-power laser beam hits the super-linear emitter, only the center of the beam profile, where the beam intensity is greatest, will spur a significant emission. That, in turn, suggests that the size of the emitting region will be smaller than the beam profile itself, resulting in sharper resolution. And the more super-linear the material is, the better the resolution will be.
But if it’s easy to state the principle of this super-linear route to super-resolution, it has proved a lot tougher to find the right super-linear nanoparticle to act as the fluorophore in a real imaging system. One reason is that many of the candidate nanoparticles, such as luminescent nanodiamonds and quantum dots, show super-linear emission only in visible wavelengths, making them less useful for imaging in strongly scattering biological systems. And previous candidates also have required complex procedures or high, potentially cell-damaging laser powers to achieve super-linear emission.
Turning to UCNPs
Like a number of other groups, the CNBP team at Macquarie, led by Denitza Denkova and Martin Ploschner, wondered if another class of nanoparticles, UCNPs, might serve as super-linear fluorophores for imaging. UCNPs are core-shell nanoparticles capable of absorbing light at near-infrared wavelengths, and then re-emitting the energy as lower-wavelength (and, thus, higher-frequency) visible and UV light. In addition to their NIR excitation wavelengths, UCNPs have another advantage: They can display super-linear emission characteristics without complex procedures or high excitation powers.
The CNBP scientists zeroed in on one particular fluorophore candidate: NaYF4 UCNPs doped with 20% Yb and 8% Tm. The team then ran a set of sophisticated computer simulations to calculate the resolution enhancement, in both the axial and lateral directions, that could be expected when imaging a given super-linear fluorophore under a given excitation beam.
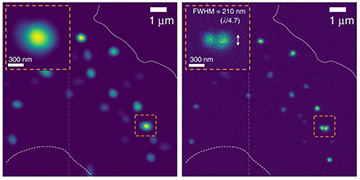
An experimental test of the system, using super-linear UCNPs embedded in nerve cells that were imaged using a standard confocal microscope, showed, according to the team, a resolution twice better (right-hand image) than confocal images at the diffraction limit (left-hand image). [Image: D. Denkova et al., Nat. Commun., doi: 10.1038/s41467-019-11603-0 (2019); CC-BY 4.0] [Enlarge image]
Next, the team tried out the system by imaging their NaYF4 UCNPs in live neuronal cells in the lab, using a conventional confocal microscope configuration. The experiment reportedly yielded images that exceeded the diffraction limit by a factor of two, in both the axial and lateral spatial directions.
Low photon budget → high resolution
One particularly intriguing facet of the CNBP team’s system is that—in contrast to other super-resolution techniques, where improving resolution requires beefing up the laser power—uSEE imaging achieves improvement in sub-diffraction imaging by lowering the photon budget. This counterintuitive finding has some significant implications for the technique’s usefulness particularly in imaging live biological samples, where high laser fluences can lead to phototoxicity and cell damage.
“Our approach works in the opposite direction to all other existing super-resolution methods,” co-lead author Denkova noted in a press release accompanying the research. “The lower the laser power, the better the resolution and the lower the risk of photo-damage to the bio-samples.” She added that, because the system requires no setup modifications or image processing, it “has the potential to enter any biological lab, at practically no extra cost.”
The team notes that, while their demonstration relied on UCNPs as super-linear emitters, the principles involved could be extended to other kinds of emitters that show super-linear behavior. Indeed, the researchers suggest that their theoretical modeling could aid in the development “novel types of super-linear emitters” that could emerge in the near term. Taken together, the authors argue, their theoretical work and lab demonstration mark “a major step toward enabling 3-D super-resolution imaging as an everyday laboratory tool.”