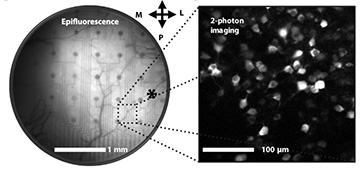
Left: A network of neurons in a mouse’s visual cortex is visible below the bilayer-nanomesh microelectrode array. Right: Excited neurons in the visual cortex captured with two-photon imaging through the microelectrode array. [Image: Yi Qiang et al., Sci. Adv., doi: 10.1126/sciadv.aat0626]
Mapping brain activity on a cellular level would allow for a detailed understanding of cognitive function, but current tandem imaging-and-electroencephalography (EEG) methods fail to produce data with this level of spatial and temporal resolution. Now, researchers from the United States have combined electrical and optical approaches to create a miniaturized and transparent microelectrode array that can capture individual neuron electrical activity and two-photon imaging of the visual cortex of awake mice (Sci. Adv., doi: 10.1126/sciadv.aat0626).
In addition to mapping brain activity, the researchers believe that their innovation could someday be used by physicians to identify real-time safe “roadmaps” for brain surgery, as well as enabling optogenetic studies on live subjects.
Old materials, new methods
Existing EEGs record the average signal for whole sections of the brain, and standard EEG arrays can block up to 82 percent of the area they cover, hiding what is happening structurally on the surface of the brain below. Previous efforts to make smaller, transparent microelectrode arrays for simultaneous cellular-scale EEG and imaging have involved graphene, but their performance suffered because of graphene’s capacitive electrode/electrolyte interface limits.
Team leads Michela Fagiolini, Boston Children’s Hospital, USA, and Hui Fang, Northeastern University, USA, discovered that they could forego graphene and use traditional microelectrode materials to produce a transparent composite microelectrode array that could record electrical activity and image on a cellular scale without sacrificing performance. The trick? A nanotechnology method called nanomeshing. Fagiolini and Fang’s team fabricated the thin bilayer microelectrode array by electroplating a transparent, conductive polymer called PEDOT:PSS (poly[3,4-ethylenedioxythiophene] polystyrene sulfonate) onto a gold nanomesh base.
The resulting microelectrode array measures 20 µm in diameter. Because they are so small, the sensors can be placed a few micrometers apart, giving them the ability to pick up the electrical signals produced by a single neuron firing. And the mesh structure offers big enough windows for high-resolution two-photon imaging of the brain’s surface below.
In addition to being small and transparent, the microelectrode arrays are also biocompatible and flexible, making them ideal for continuous use on the surface of the brain while maintaining direct contact with its bumpy surface.
In bench tests, the new arrays performed just as well as current non-transparent EEG microelectrode arrays.
Recording activity and imaging individual neurons in vivo
The researchers tested the performance of their microelectrode array in vivo with mouse models. The tiny EEG arrays were implanted in the visual cortex on the surface of the brain of adult, awake mice. They labeled neurons in the mice’s brains so that they would produce a fluorescence signal when activated by 900-nm pulsed light—the kind used in two-photon imaging.
They observed that the region of the brain underneath the microelectrode array appeared darker than the surrounding area, but it was transparent enough for a single neuron to be observed using two-photon imaging. The two-photon imaging happened at the same time that the researchers were recording electrophysiological data from the visual cortex, while the mice were shown visual stimuli. The microelectrode arrays were able to pick up signals from 300 Hz to 7 kHz in the millisecond regime. That’s sensitive and fast enough to measure the activity from a single neuron.
Future projects for the team include developing a large-scale, high-density version of their microelectrode arrays for in vivo brain recording with optical imaging for optogenetic tests, as well as penetrating microelectrode arrays for studying neuron activity below the brain’s surface.
