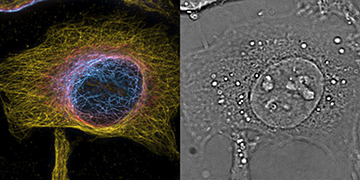
HeLa cells imaged with the PRISM system. The color view (left) encodes the z-position with one slice of the complementing 3-D phase image (right) providing cellular context. [Image: Theo Lasser / EPFL]
The past two decades have brought great advances in superresolution fluorescence microscopy, but the increase in spatial detail comes at the cost of time resolution. Many cellular processes that interest scientists occur over tiny fractions of a second, and superresolution methods cannot take images fast enough to capture these fleeting changes in living cells.
Researchers at a Swiss laboratory have combined a dynamic phase-imaging method with a 3-D fluorescence technique to make a “4-D” micro-imaging system (Nat. Photon., doi:10.1038/s41566-018-0109-4). The compound device captures 3-D images of live tissue at up to 200 Hz.
Blending two methods
Quantitative phase imaging is based on OSA Honorary Member Emil Wolf's realization, half a century ago, that the refractive index distribution from light passing through something like a cell induces a measurable phase delay. Many of the superresolution fluorescence microscopy methods based on the work of Stefan Hell, Eric Betzig and W.E. Moerner (2014 Nobel Prize in Chemistry) combine hundreds of raw images to produce a very high-resolution final picture.
At the École Polytechnique Fédérale de Lausanne (EPFL), Switzerland, Theo Lasser and his colleagues built an instrument that uses a custom image-splitting prism to acquire a stack of eight bright-field intensity images of the target cells. The eight images are recorded simultaneously but are displaced 350 nm in the z-direction, for a maximum target volume of 2.5×50×50 μm. The team developed an algorithm to extract the 3-D phase information from the image stack.
Imaging live cells
For a proof of concept, the EPFL group imaged both plastic beads of known diameters and groups of live mouse cells and live HeLa human cancer cells. The resolution of those images was approximately 350 nm in the lateral direction and 560 nm in the axial direction.
Since the heart of the EPFL microscopy system is the image-splitting prism—made by cementing together three individual optical-glass prisms along their interfaces—the team dubbed their system PRISM, for Phase Retrieval Instrument with Superresolution Microscopy.
