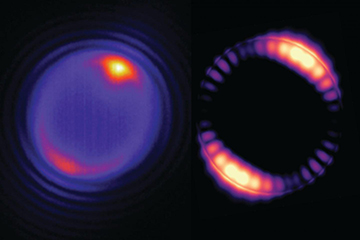
Left: A tiny bead struck by a laser (at the yellowish spot shown at the top of the image) produces optical modes that circulate around the interior of the bead (pinkish ring). Right: A simulation of how the optical field inside a 5-micron bead is distributed. [Image: Angel Fernandez-Bravo/Berkeley Lab, Kaiyuan Yao]
For today’s miniaturized electronics and minimally invasive biomedical applications, it’s important to have ever-smaller light sources that can function in confined spaces. Now, an international team led by researchers at Lawrence Berkeley National Laboratory, USA, has made a surprise discovery that allows them to create some of the smallest continuously emitting lasers yet reported (Nat. Nanotech., doi: 10.1038/s41565-018-0161-8).
The microlasers are nanoparticle-coated polystyrene beads that, at 5 µm, are smaller than red blood cells. The researchers believe that the lasers could thus find application in medical imaging and optical chip fabrication.
The trouble with small
Shrinking the size of a laser cavity increases its optical losses, meaning that greater input excitation powers are required to reach lasing thresholds. Existing miniature lasers, such as those that use lanthanide-doped upconverting nanoparticles (UCNPs), operate under pulsed excitation to avoid the optical and thermal damage that would result from these increased powers.
The authors of the new study had been exploring thulium-doped UCNPs to circumvent such issues. They predicted that nonresonant excitation of thulium-doped nanoparticles would result in efficient population inversion via an avalanche-like energy-looping mechanism. When excited at 1064 nm—a wavelength that’s resonant with excited-state absorption transitions but not with ground-state absorption, and that has the additional benefit of being transmitted efficiently through tissue—thulium-doped sodium yttrium fluoride nanoparticles exhibit upconverted luminescence in the visible and near infrared.
Whispering-gallery modes
At the same time, the researchers were also looking into the potential of polystyrene beads for brain imaging. Polymers are attractive for biological applications because they are biocompatible or biodegradable. The authors knew that larger microspheres of bulk upconverting material had shown low-power lasing by virtue of whispering-gallery modes, in which light interferes contructively on closed-loop paths via total internal reflection within the microsphere surface.
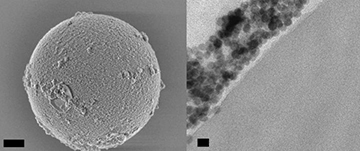
SEM image of a 5-micron-diameter polystyrene bead coated with nanoparticles (left; scale bar 1 micron), and TEM image showing a cross-section of a bead, with nanoparticles along its outer surface (right; scale bar 20 nm). [Image: Angel Fernandez-Bravo/Berkeley Lab]
Putting these technologies together, the researchers coated the polystyrene beads with the thulium-doped UCNPs. Upon nonresonant excitation, the beads emitted periodic spikes of light at very specific wavelengths—because the upconverted light emitted by the nanoparticles was bouncing around inside the bead in a whispering-gallery fashion and, in turn, stimulating the emission of yet more light from the nanoparticles.
The threshold for this lasing behavior is lower than that reported for other UCNP-based lasers, so the new lasers can operate continuously for hours at lower powers than before. In fact, the researchers have demonstrated stable lasing at blue and near-infrared wavelengths for more than five hours.
In addition, the researchers anticipate being able to tune the emitted light by changing the size and composition of the beads. The 5-μm polystyrene cavities support both transverse electric and transverse magnetic propagating modes, and the number and wavelength of the amplified modes can be selected by changing the diameter of the microspheres.
Operation in vivo
To be suitable for in vivo biosensing applications, the microlasers need to operate in aqueous environments. The researchers discovered that after dipping the beads in blood serum, which coated them in proteins, they would lase in water as well as in air. As a result, the beads may enable detection of small changes in temperature or pressure in biological systems, because of the sensitivity of the lasing modes to the cavity shape and size. The authors have also shown that the beads can be trapped and steered with the same laser beams that are used to excite them.
Co-lead author James Schuck, now at Columbia University, emphasized in a press release on the work that this was an unexpected yet fortuitous result. “We just happened to have the right nanoparticles and coating process to produce these lasers.”
