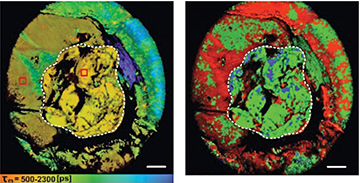
Fluorescence time-resolved images of NAD(P)H in a whole tumor in vivo using a new macro-FLIM imaging system. The border of the tumor is marked with a white line. Scale bar: 2 mm. [Image: V.I. Shcheslavskiy et al., Opt. Lett. 43, 3152 (2018)]
Fluorescence lifetime imaging microscopy (FLIM) is a common tool in cell biology, but its use is limited by a small, 1-mm field of view (FoV). Now, scientists from Becker & Hickl GmBH, Germany, and Privolzhskiy Research Medical University, Russia, have designed and demonstrated a scaled-up FLIM-based imaging system with an 18-mm FoV and lateral resolution of 15 µm (Opt. Lett., doi: 10.1364/OL.43.003152).
The research team says its new “macro” system could make FLIM imaging technology available for biomedical applications, such as detecting the border between healthy tissue and a cancerous tumor during surgery or observing biological processes on a cellular level. In demonstrations with their confocal microscopy-based macro-FLIM system, the researchers report imaging a whole tumor in vivo with cellular resolution and high metabolic specificity—a first, they say, for a FLIM-based imaging system.
A bigger window
Current FLIM systems use a scanning laser to obtain millimeter-sized high-resolution images of biological samples. The images are created by measuring how long it takes for the light from fluorescent molecules or tags in the sample to decay. Fluorescence decay rates vary depending on the environment—for example, differences in temperature, pH, refractive index and viscosity. Thus fluorescence decay-rate measurements not only provide structural information about the sample, but offer a glimpse at the sample’s microenvironment.
For the new FLIM system, the researchers swapped the traditional scanning laser for diode lasers with picosecond-length pulses. They also added sensitive and fast fluorescence detectors and time-correlated single-photon counting (TCSPC) technology. These improvements combined with “careful optical design” enabled Vladislav I. Shcheslavskiy and his colleagues to achieve a FoV of 18 mm with a lateral resolution of 15 µm.
To create images using the macro-FLIM system, the researchers place a sample in the intermediate image-plane of the confocal scanner and use TCSPC technology to record and plot photon distribution in relation to the timing and position of the laser pulses across several image planes.
In vitro and in vivo imaging
Shcheslavskiy’s team demonstrated the cellular resolution of the macro-FLIM system by imaging yellow-green fluorescent microbeads with a diameter of 14.6 µm and fluorescently labeled cancer cells in vitro.
![]()
The macro-FLIM system might one day find use in the clinic as a sensitive and precise method for identifying the edges of tumors during surgery. [Image: V.I. Shcheslavskiy]
The researchers demonstrated the macro-FLIM system’s use in vivo by imaging a whole tumor (7 mm x 8mm in size) in a live mouse model. By simultaneously measuring fluorescence decay-rates of nicotinamide adenine dinucleotide (phosphate) NAD(P)H (a fluorophore that assists with cell metabolism and can indicate cancer) and mKate2 (a genetically encoded red fluorescent protein that tags living cancer cells), they were able to map the tumor’s border on a cellular level and also observe the tumor’s NAD(P)H metabolic activity.
In addition to imaging NAD(P)H and mKate2 signals, the researchers say that their system could also be used to investigate other tumor activities on a macroscale, including both metabolism and apoptosis (cell death) or metabolic and oxygen status. And, if combined with a translational stage, the macro-FLIM system could in theory image samples up to 10 cm2.
