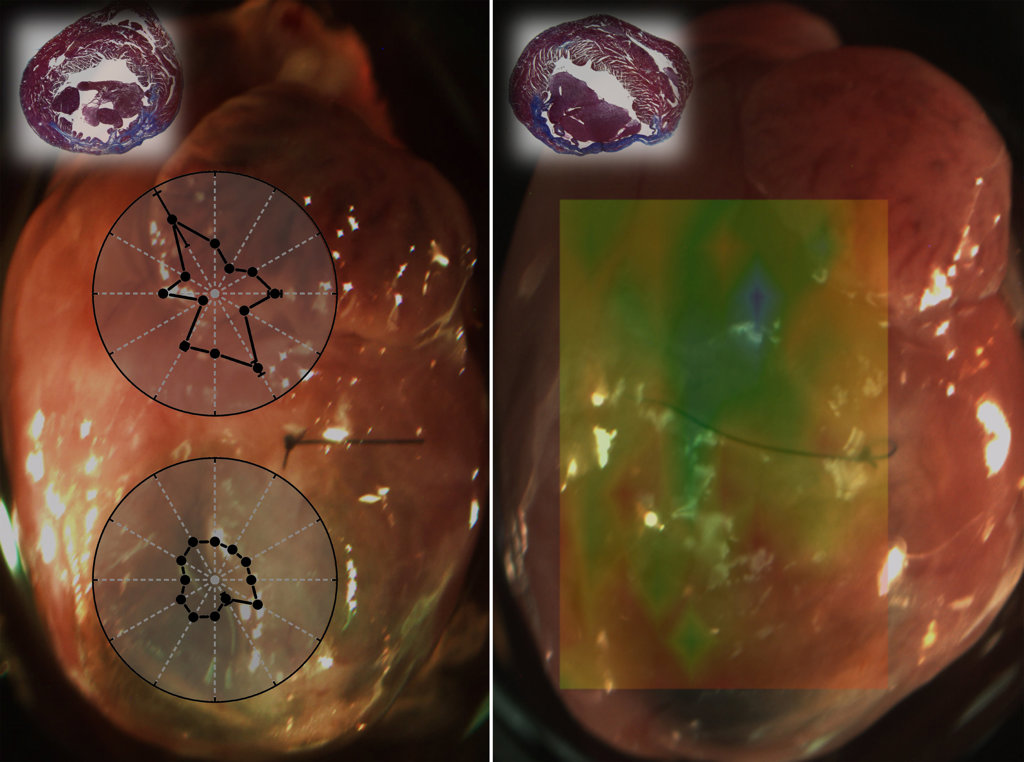
Overlays on the left show direction-dependent differences between healthy (top) and damaged heart tissue (bottom) from a mouse myocardial infarction (MI) model. The overlay on the left shows spatially resolved heart-tissue stiffness measurements from a mouse MI model. The green shows healthy tissue; the yellow and red areas show damaged tissue. [Image: Kirill V. Larin / University of Houston]
A research team led by Kirill Larin, University of Houston, USA, and James Martin, Baylor College of Medicine, USA, developed and tested an imaging method based on optical coherence tomography (OCT) to measure biomechanical changes in damaged heart tissue in mice post-myocardial infarction (MI) on a small, sub-organ scale (Biomed. Optics Express, doi: 10.1364/BOE.9.000728).
The system, called optical coherence elastography (OCE), uses puffs of air to generate waves in a tissue sample. The tissue’s mechanical properties are revealed by analyzing the speed and spatial characteristics of the waves as they travel through the sample. The researchers hope that their OCE system will be used in live mouse models to help develop and test new therapies for healing heart tissue damaged during MIs.
Mouse models
During an MI, the heart is deprived of oxygen, which causes tissue damage and scarring. This scar tissue is stiff and reduces the heart’s ability to pump blood. Mouse models are important tools for studying MI because the animal model has well-established genetic tools for investigating and manipulating tissue biomechanics and regeneration. However, viewing these biomechanical changes is difficult because existing imaging methods, like MRI, are better suited for observing whole organs or large animal models than the small scale required for observing mouse hearts.
For OCE to work in small tissues, like a mouse heart, Larin and Martin’s team designed the system to allow for precise timing and application of small-amplitude, non-damaging waves in the tissue. The OCE system is made up of an air-pulse loading unit and an OCT imaging unit. The air pulses are tailored to be delivered at pressures low enough to ensure minimal damage to the tissue sample. The OCT imaging unit uses a Ti:Sapphire laser with an 810-nm central wavelength and a 110-nm bandwidth. A fiber-based Michaelson interferometer was added for light interference. The entire system has an axial resolution of about 5 µm in tissue and a transverse resolution of about 4 µm.
Measuring tissue elasticity with OCE
The researchers used two methods to test their OCE system’s ability to differentiate between scarred and healthy heart tissue in mouse MI models: direction-dependent elastic-wave assessment and spatially resolved displacement damping.
For direction-dependent elastic-wave assessment of healthy and MI-damaged heart tissue, the samples were placed on a manual rotation stage, and elastic wave-propagation was measured in six directions at 30-degree intervals. For the spatially resolved damping analysis, the samples were placed on a 3-D motorized linear stage, and the OCT imaging beam was co-focused with air-pulse stimulation.
Compared with healthy heart tissue, the tissue from the MI hearts had reduced elastic-wave velocity and less mechanical anisotropy—in simpler terms, the scarred MI heart tissue was stiffer and structurally less organized than the healthy tissue. Histological observations conducted after OCE testing verified the researchers’ findings, leading them to conclude that OCE could be used for non-destructive biomechanical characterization of MI in mouse models.
