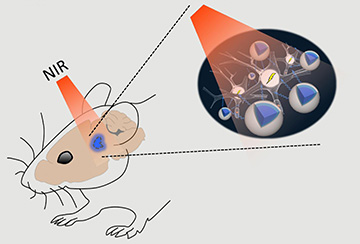
Near-infrared light applied above the skull can easily pass through brain tissue with minimal scattering and reach deep structures. Up-conversion nanoparticles (UCNPs; blue) absorb this light and emit visible light to activate light-sensitive channels expressed in nearby optogenetically modified nerve cells. [Image: RIKEN]
Researchers have developed and tested a method to non-invasively manipulate optogenetically modified nerve cells with blue light deep within the brain in live mouse models (Science, doi: 10.1126/science.aaq1144).
The blue light used for optogenentic manipulation has a wavelength that scatters easily in brain tissue, preventing it from reaching areas beneath the skull without the help of invasive fiber-optic probes. To overcome this depth limitation without using probes, the team, led by Thomas McHugh at the RIKEN Brain Science Institute in Japan, developed up-conversion nanoparticles (UCNPs) that can be embedded in the brain and transform low-energy near-infrared (NIR) light from a source outside the skull into the blue wavelengths needed to optogenetically activate or inhibit nerve cells.
Although much research remains to be done on this method before it’s used in humans, the team hopes to someday see non-invasive UNCP-mediated optogenetics used in deep-brain stimulation therapy to treat a variety of neurological conditions, including seizures, severe depression, Alzheimer’s and paralysis.
Non-invasive optogenetics with depth
Scientists use nerve cells with genetically modified light-responsive sodium-ion (Na+) channels to study brain activity. By shining blue light on nerve cells with these modified ion channels, they can either increase intracellular Na+, which triggers a nerve impulse, or prevent an increase in intracellular Na+, which inhibits a nerve impulse. Currently, this blue “go” signal is delivered to deep-brain regions via fiber-optic probes. These probes, although very thin, are invasive and can potentially cause tissue damage. McHugh and his colleagues believe that their UCNPs will remove the need to use potentially harmful probes to deliver blue light deep within the brain.
The new UCNPs are biocompatible, thanks to a silica coating, and are doped with lanthanide-family elements. These elements have an optogenetic actuation that can be tuned to receive and then convert low-energy NIR light to higher-energy blue light. Even though this up-conversion releases energy, the researchers say it doesn’t generate enough heat to cause tissue damage. During preliminary tests with UCNPs, they observed only a minimal increase in local temperature and no cellular damage in the surrounding tissue.
In vivo neural activation and inhibition
For demonstrations of their non-invasive optogenetics technique, the researchers used live mouse models and injected UCNPs into different regions of the brain containing nerve cells with light-responsive Na+ channels.
First, the scientists confirmed that the UNCPs could up-convert NIR wavelengths from a laser source outside the skull into visible blue light by placing an optic fiber nearby to record the blue-light emission. Next, the researchers triggered neural activation responses (released dopamine) and inhibition responses (silenced seizures and synchronized brain waves) in three different live mouse models. In a fourth mouse model, they triggered learned memories that could be recalled by the mice two weeks after initial UNCP implantation.
In a press release, McHugh said the UNCPs “effectively extend the reach of our lasers, enabling the ‘remote’ delivery of light and potentially leading to non-invasive therapies.”
McHugh’s research team includes scientists from RIKEN as well as the National University of Singapore, the University of Tokyo and Keio University in Japan, and Johns Hopkins University in the United States.
