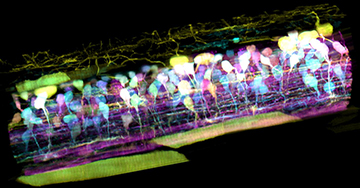
Inside the spinal cord of a zebrafish embryo, new neurons light up in different colors, letting scientists track nerve circuit development. [Image: T. Liu et al. / Science 2018]
To really understand cellular and subcellular dynamics, you need to be able to observe reactions to environmental cues while they happen in live organisms. Now, U.S. researchers from Howard Hughes Medical Institute (HHMI), Boston Children’s Hospital and Harvard Medical School report designing and demonstrating a new way of observing in vivo subcellular dynamics in 3-D video using adaptive optics (AO) and lattice light-sheet microscopy (LLSM) (Science, doi: 10.1126/science.aaq1392).
The AO-LLSM microscope, say the scientists—led by 2014 Nobel Laureate in chemistry Eric Betzig, HHMI—can image across large multicellular volumes and create noninvasive aberration-free videos of subcellular processes, including endocytosis (a cell engulfing large particles), intracellular remodeling during cell division, and the migration of nerve cells, immune cells and metastatic cancer cells in vivo.
Applying astronomy’s “guide star” to live microscopy
The AO-LLSM microscope borrows one of astronomy’s tools for obtaining brilliantly clear telescopic images of planets and galaxies through our optically hazy atmosphere: The AO guide star. Instead of shining a laser into the heavens, Betzig and his team shine a two-photon excited fluorescence (TPEF) guide star over a live biological specimen. This guide star, similar to its astronomical cousin, acts as a reference—the same sample-induced aberrations affecting the image of the guide star within the specimen are also distorting planar images of the specimen acquired by LLSM. The guide-star aberrations are measured and corrected with a phase-modulation element that applies an equal but opposite distortion.
However, LLSM images are susceptible to two types of aberrations, because the light sheet travels through the imaged specimen for excitation as well as detection. To correct excitation and detection aberrations, the team employed two independent AO systems (that is, two AO channels), one for the excitation arm and one for the detection arm of the microscope.
After capturing and correcting the LLSM planar images, the system computationally stitches them together to create sharp 3-D videos of the specimen. For imaging specimens with fields-of-view (FOVs) greater than 30 µm to 60 µm, the researchers employed a tiled acquisition method to compensate for AO correction variation in both space and time. Here, each image subvolume “tile” has its own AO correction before being computationally stitched together.
The researchers say that their new technique creates 3-D images at resolutions that exceed nominally higher numerical-aperture confocal or spinning-disk microscopes. And, because the specimen is scanned plane by plane, potentially damaging photon exposure is reduced compared to point-imaging techniques.
Demonstrating the AO-LLSM microscope in vivo
The researchers used zebrafish embryos in many of their demonstrations because they are nearly transparent, making them easier to image, and also because they can be used as genetic models for human diseases. In addition to observing metastatic human breast cancer cells migrate through living tissue in a zebrafish embryo, the team has also used the AO-LLSM microscope to view organelle dynamics in a zebrafish brain, cell-membrane dynamics in a zebrafish eye and neural-circuit development in a zebrafish spinal cord. The following link goes to an AO-LLSM video of an immune cell migrating inside a zebrafish embryo’s inner ear while scooping up particles of sugar along the way: https://youtu.be/Hz0VlUVjYfI.
The researchers successfully demonstrated their tiled acquisition for aberrations in large FOV scans using the tail of a zebrafish embryo and imaging volumes that measured 213×213×113 µm, which translated to 7×7×3 tiles.
Caveats and future plans
Authors of the Science paper note that while their AO-LLSM microscope performed well during demonstrations, their results come with several caveats. One of the biggest challenges they identified is mining the huge amount of complex data generated for as much detail as possible about the imaged specimen. In addition, only weakly scattering specimens (that is, specimens that are close to translucent), like zebrafish embryos, can be imaged using AO-LLSM because the fluorescence induced by the light sheet is captured with wide-field optics.
The existing AO-LLSM setup takes up about 10 feet of space on a lab bench. Betzig says his team is working on a next-generation version that should have a much smaller footprint and have a price tag that’s within the reach of individual labs. The plan for now is to house one of these next-generation AO-LLSM instruments at HHMI’s Janelia Advanced Imaging Center, where scientists from around the world can apply to use it.
