![]()
[Image: Getty Images]
More than half of all U.S. surgical procedures are of the “minimally invasive” type, but surgeons still rely on the qualitative senses of vision and touch for guidance. Optical scientists are working to provide physicians with more quantitative information, such as blood oxygenation levels, for the tissues that lie deep within the patient’s body.
A technique called three-dimensional single snapshot of optical properties (3D-SSOP)—developed by the research group of Sylvain Gioux at the Beth Israel Deaconess Medical Center/Harvard Medical School, under funding from the U.S. National Institutes of Health—could add some of that real-time data, according to Joseph P. Angelo, now a postdoctoral fellow at the U.S. National Institute of Standards and Technology (NIST), who gave an invited talk at a Frontiers in Optics 2017 session on biomedical imaging.
Stripes of light
The 3D-SSOP technique grew out of a desire to improve on spatial frequency-domain imaging (SFDI), which measures the spectral frequency response of tissue to structural illumination (basically, stripes of light). Scientists have been developing medical imaging systems based on SFDI, but Angelo says that 3-D SSOP has several advantages.
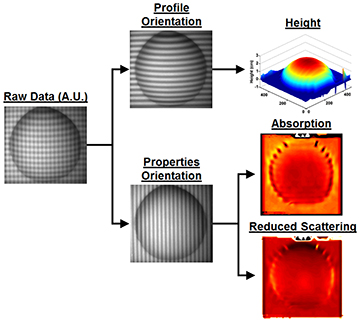
How 3D-SSOP works: A dual sinusoidal wave is projected onto a specimen. One wave is sensitive to the specimen’s optical properties and the other to the specimen’s profile. [Image: Martijn van de Giessen et al., Biomed. Opt. Express, doi: 10.1364/BOE.6.004051]
For one, SFDI needs to analyze the reflected light from two projected patterns—one AC pattern and one DC (planar illumination) pattern—at two spatial frequencies, according to Angelo. To make the image three-dimensional, a third pattern must be analyzed.
However, SSOP takes advantage of the inherent DC offset of an AC pattern of light, according to Angelo. “This means that with processing techniques, you can use a single sinusoidal pattern and get a DC and AC image,” he says. “Furthermore, 3D-SSOP combines the profilometry image as well, so now three patterns are analyzed from a single image.”
Only one image needed
While standard SFDI processing requires three-phase demodulation for each of the three projected profiles (DC, AC, and 3-D profile), 3D-SSOP demodulation uses Fourier filtering and Hilbert transforms onto a single projected pattern, Angelo says. Thus, three-dimensional SFDI requires nine images to produce one frame, while 3D-SSOP needs only a single image.
Angelo and his colleagues in Gioux’s group came up with a system that takes less than 30 ms to acquire and process each image. That speed supports a potential frame rate of 33 fps, well above the team’s goal of 24 fps.
With this type of system, surgeons could test blood oxygenation levels in particular regions of a patient’s body, rather than relying on the global pulse oximeter clipped to the patient’s finger. For instance, while surgeons are performing a tissue graft or limb reattachment, the detector could pinpoint small regions of skin or muscle that are not getting sufficiently oxygenated blood and thus fix the circulatory problem in real time. During the talk, Angelo showed a short 3D-SSOP video of his hand, revealing the fingers’ lack of oxygenation while he had his upper arm squeezed tight in a blood-pressure-measuring cuff.
Gioux’s group, now at the University of Strasbourg, France, continues to pursue improvements to 3D-SSOP toward the technique’s validation during surgical interventions in humans, under the funding of the European Union ERC program.
Other biomedical technologies
Several other FiO 2017 sessions covered the range of biomedical optics applications from cancer diagnosis to imaging nanoscale details of live cells. For example, Angela Harrivel of NASA Langley Research Center, USA, described how the space agency would like to use functional near-infrared spectroscopy and adaptive noise filtering to study how people pay attention to operational tasks.
A team based at the University of São Paulo, Brazil, investigated the use of fluorescence lifetime imaging microscopy to identify tumor tissue by the length of the decay of its fluorescence. The researchers tested their methods on skin lesions as a proof of concept but want to extend the method to cervical cancer diagnostics. A group at Samara State University, Russia, described how a low-cost Raman spectrometer could be used as a melanoma screening tool for rural patient populations who lack access to dermatologists and oncologists.
Corrected on 6 October, 6:20 a.m. EDT, to clarify differences between the 3D-SSOP and SFDI techniques and the roles and funding sources of the researchers that did the work.
