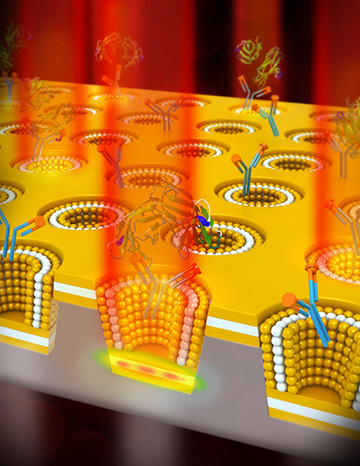
Plasmonic sensing of a cancer biomarker is enhanced by a plasmonic nanocup metal-insulator-metal cavity design. [Image: University of Illinois]
Researchers from the University of Illinois at Urbana-Champaign (USA) have developed a new plasmonic refractive-index (RI) sensor using what they call a multilayer nano Lycurgus cup array (ML-nanoLCA). During laboratory demonstrations, the team reports, its plasmonic RI-sensing device outperformed current surface plasmon resonance (SPR) systems by two orders of magnitude. That advantage, according to the scientists, could help the sensor find eventual use in clinical settings to improve detection of disease biomarkers in the blood (Adv. Opt. Mater., doi: 10.1002/adom.201601051).
Inspired by ancient Rome
The ML-nanoLCA is comprised of an insulating layer of cadmium sulfide (which has a high RI and a low extinction coefficient in the visible range), sandwiched between two layers of gold that provide strong confinement of the electromagnetic field on the nanoscale. This metal-insulator-metal sheet is punctuated with 500-nm deep nanocups with 85-degree sidewall angles. Light entering the nanocups is amplified in the nanocavity, increasing the transition-signal intensity before it’s transmitted out and picked up by the photonic sensor. The name of the device is inspired by the celebrated Lycurgus cup of ancient Rome, which—due to a unique nanostructure in the glass—appears to change color under different lighting conditions.
During optical-property characterization studies of the ML-nanoLCA sensor, the researchers found that an increase in the concentration of biomolecules on the surface of the device resulted in an increase in RI, which in turn produced an increase in transmission signal intensity with no shift in the transmission peak wavelength. They also found that the sensor contains a wavelength region in which the signal intensity remains constant when RI is varied. That means the device has the potential to act as its own reference sample, which could be particularly useful in environments outside a controlled laboratory setting.
Demonstrating biomarker detection
To test the ML-nanoLCA’s ability to detect biomolecules in solution, the researchers peppered the device’s surface with immobilized antibodies to carcinoembryonic antigen (CEA)—a biomarker for several types of cancers. After incubating the device in solutions with different CEA concentrations, they applied a light-emitting diode (LED) to the plasmonic sensor and used a photodetector to record changes in light transmission intensity. They report that these demonstrations showed that the sensor could detect 1 ng of CEA per milliliter—a limit of detection two orders of magnitude better than SPR sensing systems.
In addition to outperforming SPR sensing systems, the researchers say their ML-nanoLCA sensor requires less instrumentation—an LED light source instead of a laser, and a photodetector instead of a sensitive spectrometer—which reduces the device’s footprint and cost, making it potentially more suitable for point-of-care diagnostics and use in low-resource areas.
