
Unlike most substances, liquid water is denser than its solid phase, ice. [Image: Stockholm University]
In two different X-ray investigations, researchers have dug into some of the exotic properties of that most familiar of substances—water.
In one study, researchers from Sweden, Japan and South Korea used a femtosecond X-ray laser to investigate the behavior of evaporatively supercooled liquid water, and to confirm the long-suspected view that water at low temperatures can exist in two different liquid phases (Science, doi: 10.1126/science.aap8269). In the other, a U.S.-Japanese team used high-resolution inelastic X-ray scattering to probe the dynamics of water molecules and how the liquid’s hydrogen bonds contribute to its unusual characteristics (Sci. Adv., doi: 10.1126/sciadv.1603079).
Burst pipes and floating cubes
Anyone who has confronted a burst water pipe on a frozen winter morning has firsthand knowledge of one of H20’s unusual characteristics. Whereas most substances increase in density as they go from a liquid to a solid state, water reaches its maximum density at 4°C, above its nominal freezing point of 0°C. That’s also the reason that the ice cubes float at the top of your water glass rather than sinking to the bottom.
Grappling with this anomalous behavior, a research team at Boston University suggested around 25 years ago, based on computer simulations, that in a metastable, supercooled state, water might actually coexist in two liquid phases—a low-density liquid and a high-density liquid. Those two phases, the researchers proposed, merged into a single phase at a critical point in water phase diagram at around –44°C (analogous to the better-known critical point at a higher temperature between water’s liquid and gas phases).
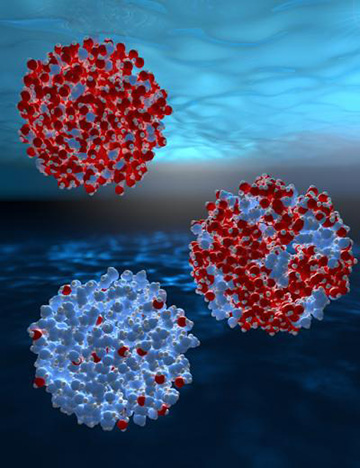
Experiments using femtosecond X-ray free-electron lasers illuminated fluctuations between two different phases of liquid water—a high-density liquid (red) and a low-density liquid (blue)—as a function of temperature in the supercooled regime. [Image: Stockholm University]
Actually getting liquid water to that frigid point has, however, seemed a bit of a pipe dream. While very pure liquid water can be rapidly supercooled to temperatures moderately below 0°C relatively easily, the proposed critical point lies far below that temperature range, in what researchers have dubbed a “no-man’s land” in which ice crystalizes much faster than the timescale of conventional lab measurements.
Leveraging ultrafast lasers
To move past that barrier, a research team led by Anders Nilsson of Stockholm University, Sweden, turned to the rapid timescales enabled by femtosecond X-ray free-electron lasers (XFELs). At XFEL facilities in Korea and Japan, the team sent a stream of tiny water droplets (approximately 14 microns in diameter) into a vacuum chamber, and fired the XFEL at the droplets at varying distances from the water-dispensing nozzle to obtain ultrafast X-ray scattering data.
The tiny size of the droplets meant that as they traveled through the vacuum they rapidly evaporatively cooled—with the amount of cooling related to the time they spent in vacuum under a well-established formula. Thus, by taking X-ray measurements at varying distances from the nozzle, the researchers could examine the structural behavior of the liquid water at multiple temperatures in the deep-supercooling regime, near the hypothesized critical point. “We were able to X-ray unimaginably fast before the ice froze,” Nilsson said in a press release, “and could observe how it fluctuated” between the two hypothesized metastable phases of liquid water.
The experiments allowed the team to flesh out the phase diagram of liquid water in a supercooled region previously thought to be inaccessible to experiment. And the researchers believe that the use of femtosecond XFELs to probe thermodynamic functions and structural changes at extreme states “can be generalized to many supercooled liquids.”
Illuminating water’s dynamics

A team led by scientists at the U.S. Oak Ridge National Laboratory used inelastic X-ray scattering to visualize and quantify the movement of water molecules in space and time. [Image: Jason Richards/Oak Ridge National Laboratory, US Dept. of Energy]
A second set of experiments, from researchers at the U.S. Oak Ridge National Laboratory, the University of Tennessee, and the SPring-8 synchrotron laboratory in Japan, looked at water’s dynamics at room temperature, using inelastic X-ray scattering (IXS). Here, the research team was interested in sussing out how water molecules interact in real time, and how the strongly directional hydrogen bonds of water molecules work together to determine properties such the liquid’s viscosity.
The researchers illuminated these dynamics through a series of experiments in which they trained radiation from the SPring-8 facility’s high-resolution IXS beamline, BL35XU, onto a 2-mm-thick sample of liquid water. Through multiple scattering measurements across a range of momentum and energy-transfer values, the team was able to build a detailed picture of the so-called Van Hove function, which describes the probability of interactions between a molecule and its nearest neighbors as a function of distance and time.
The team found that water’s hydrogen bonds behave in a highly correlated fashion with respect to one another, which gives liquid water its high stability and explains its viscosity characteristics. And, in a press release, the researchers further speculated that the techniques used here could be extended to studying the dynamics and viscosity of a variety of other liquids. Some of those studies, they suggested, could prove useful in “the development of new types of semiconductor devices with liquid electrolyte insulating layers, better batteries and improved lubricants.”
