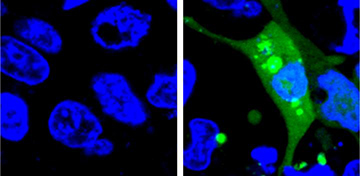
Image of cells expressing the AgHalo sensor before (left) and after (right) cellular stress. [Image: Courtesy of Yu Liu, Penn State University]
Two research groups have reported new approaches that use fluorescent sensor proteins to evaluate drug safety and drug uptake inside living cells. In one study, a U.S. team demonstrated use of a fluorescent sensor to quantifiably measure drug-induced proteomic stress, which is associated with drug toxicity. In the other, a Swiss-German group developed two Förster resonance energy transfer (FRET)-based ratio-metric biosensors that measured drug uptake into live cells in real-time and with spatiotemporal resolution.
Capturing drug-induced stress
Led by Xin Zhang, a team of chemists from Pennsylvania State University, USA, report creating and demonstrating a fluorogenic sensor that can detect and quantify proteome stress in the misfolding of intracellular proteins (Angew. Chem. Int. Ed., doi: 10.1002/anie.201702417).
Drug-induced proteome stress is an indicator of drug toxicity since misfolded and aggregated proteins cannot perform their necessary functions inside the cell. The researchers say their sensor is the first fluorescent sensor that doesn’t light up until protein misfolding and aggregation begins. Other fluorescent sensors constantly emit light, with the light becoming brighter when fluorescently tagged misfolded proteins glom together.
Water trigger. The key element of the U.S. team’s sensor is an aggregation-prone Halo-tag mutant protein, dubbed AgHalo. AgHalo has a fluorogenic ligand in the hydrophobic region of the molecule, where it is unlikely to encounter water. If AgHalo is stressed from an internal (e.g., heat) or external (e.g., drugs) force, the protein misfolds and aggregates, exposing the fluorogenic ligand in the hydrophobic region to the cell’s water-filled cytoplasm. This causes the fluorogenic ligand to interact with the water and light up. The strength of the fluorescent signal indicates the degree of overall intracellular protein misfolding and aggregation since the sensors only emit light when AgHalo’s structure is compromised.
After evaluating the AgHalo sensor’s ability to fluoresce under heat-induced proteome stress, the team demonstrated the AgHalo sensor in live cells incubated with five commonly used anti-cancer drugs. While none of the five drugs caused significant levels of proteome stress, all produced a detectable level of stress, evidenced by AgHalo fluorescence. These results aren’t a sign that these drugs may not be safe, says the team, but that evaluating the drugs with the new fluorogenic sensor could help in improving the safety of existing drugs and in developing new, safer medications.
Measuring drug uptake
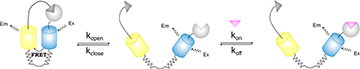
Design principle of the FRET sensor proteins to evaluate cellular uptake of drugs and drug candidates. [Image: ACS Sens., doi: 10.1021/acssensors.7b00331] [Enlarge image]
Across the Atlantic, researchers from Switzerland and Germany are converting protein drug targets into fluorescent sensors to evaluate how well living cells actually absorb drug candidates (ACS Sens., doi: 10.1021/acssensors.7b00331). Led by Kai Johnsson of the Max-Planck-Institute for Medical Research in Germany, the European team created and demonstrated two fluorescent sensor proteins for evaluating the cellular uptake of two drugs.
The first is an inhibitor of the enzyme human carbonic anhydrase II (HCAII), which is used as a diuretic and for the treatment of glaucoma and epilepsy. The second is an inhibitor of protein-protein interactions (PPIs) between tumor suppressor p53 and human double minute 2 (HDM2), which is being tested in clinical trials for the treatment of human cancers.
According to Johnsson and his colleagues, there is a shortage of assays that measure intracellular drug concentrations in vivo with spatiotemporal resolution. Their method, which uses FRET-based ratio-metric biosensors to measure drug concentrations within the cell, is meant to be used along with existing medium- and high-throughput in vitro assays to improve evaluation and measurement of cellular uptake and membrane permeability.
Binding ligands to drug targets. The Johnsson team’s sensor consists of a drug target, two FRET-capable fluorescing proteins and a ligand that binds to the drug target. When the sensor is not in an environment with the drug, the ligand binds to the target, bringing the two fluorescing proteins closer together, which is observed via microscope as an increase in FRET. When the sensor is exposed to a high concentration of the drug, the drug molecule displaces the ligand, pushing the fluorescing proteins further away and decreasing FRET. Analysis of the FRET ratios can provide information about real-time drug concentration with temporal resolution.
Demonstrations with live cells expressing the HCAII-based sensor and the p53-HDM2-based sensor were in line with previously published results using in vitro assays of the same inhibitors. The researchers say that the modular design of their biosensors can be adapted to other molecules of interest to the pharmaceutical industry, as well to improve the efficacy of drugs currently on the market.