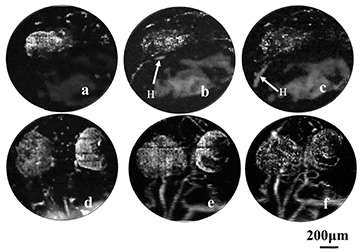
Researchers from the University of Electronic Science and Technology of China used photoacoustic imaging to provide a label-free view of vascular development in the heart (H) and brain/eye (E) regions of zebrafish embryos. Images a to c show the heart at 22, 25 and 28 hours after fertilization; images d to f show the microvascular development in the brain at 36, 48 and 72 hours after fertilization. [Image: Chen et al., Biomed. Opt. Express, doi: 10.1364/BOE.8.002359] [Enlarge image]
A research team in China has exploited photoacoustic imaging to track development of the circulatory system in zebrafish embryos, without the use of labels or contrast agents that can gum up the view of sensitive biological processes (Biomed. Opt. Express, doi: 10.1364/BOE.8.002359). The researchers believe that this proof of concept in one of biomedicine’s central model organisms could open a path in the future toward using the technique to study human cardiovascular diseases, as well as brain diseases tied to blood-flow issues.
Avoiding labels
The zebrafish’s transparent embryos, well-understood genome and rapid growth pattern have made it a preferred model organism for tracking development and studying developmental disorders. But in imaging the intricate internal growth of the fast-developing fish, high-resolution optical techniques such as confocal microscopy and two-photon microscopy have run aground on several problems in the past. Perhaps the biggest is the need to use a fluorescent label or contrast agent, which in this system can easily interfere with the delicate biological processes under study. And, of course, there’s the perennial problem of optical scattering in biological tissues, which lowers signal quality and penetration depth.
The research team, led by Lei Xi of the University of Electronic Science and Technology of China, sought to get around these problems by leveraging a different approach, photoacoustic imaging. In this method, light plays an indirect role in obtaining an image. When a non-ionizing, low-power laser pulse hits a bit of biological tissue, a small amount of its energy is converted to heat; the resulting thermoelastic expansion creates ultrasound waves that can be picked up by transducers, analyzed and converted into images.
Because the laser pulse can be tuned to the specific absorption characteristic of biomolecules such as hemoglobin, an externally applied contrast agent or fluorescent label is unnecessary. And the use of ultrasound for gathering the image avoids some of the problems of optical scattering endemic to biological tissue.
Laser to tank to transducer
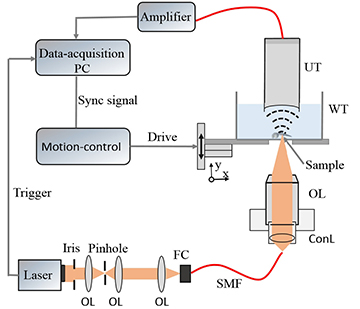
The team’s experimental setup (OL, objective lens; FC, fiber coupler; WT, water tank; UT, ultrasound transducer). [Image: Chen et al., Biomed. Opt. Express, doi: 10.1364/BOE.8.002359] [Enlarge image]
In the Chinese team’s setup for transmission-mode optical resolution photoacoustic microscopy (ORPAM), 5-ns pulses from a 532-nm laser were filtered, shaped, collimated and passed through a single-mode optical-fiber link to be focused with an objective lens—a 4× lens to image an entire fish embryo, and a 10× to drill down to deeper cardiovascular structures. The team then trained the focused beam on a zebrafish embryo lying at the bottom of a water tank. The resulting photoacoustically derived sound waves, propagating through the tank, were picked up by an ultrasound transducer at the top of the tank, and sent to a computer for processing into images.
The team found that the setup allowed it to image blood vessel development across an entire fish embryo with 3.5-micron resolution using the 4× objective—and to track the development of brain and heart vasculature with 1.5-micron resolution using the 10× lens. Moreover, they were able to nail down the exact timing of the development at a range of time slices, providing a whole-body view of vessel development in the embryos.
Speeding up
Right now, the technique’s Achilles’ heel seems to be timing: using the initial setup, it took some 25 minutes to obtain a whole-body image, an awkward duration when dealing with squirming, living fish embryos. The researchers believe they can improve the technique’s speed with a better preamp, which would allow for faster time averaging.
When those timing issues are ironed out, however, the technique could prove useful in a wide variety of settings, in the view of team leader Xi, who sees potential in studies of any of a variety of diseases related to blood circulation, including epilepsy, heart disease, atherosclerosis, hypertension and more. Further, the group notes in the paper, multimodal setups combining ORPAM with another technique, optical coherence tomography, could provide a still more detailed structural view. And the team believes that combining all of these developments on the imaging side with the rich store of genetic information on zebrafish development could allow for new insights on the genetic roots of vascular diseases.