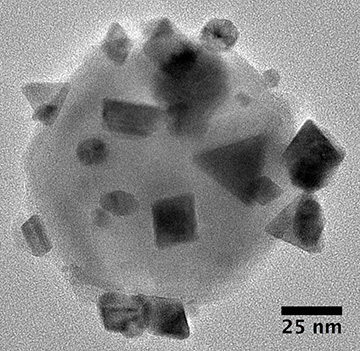
In the “antenna-reactor” catalysts devised by the Rice University team, islands of reactive palladium, a highly efficient catalyst at high temperatures, dot the aluminum oxide surface of an underlying aluminum crystal. The crystal serves as a plasmonic antenna to capture light and activate the catalytic islands. [Image: D. Swearer/Rice University]
For years, industrial chemists have dreamed of replacing energy-intensive catalytic chemistry, which lies at the heart of many key manufacturing processes, with photocatalysis—low-energy catalysis driven by sunlight. The rub has been that the best catalysts are notoriously poor at harvesting solar energy.
Now, a team at Rice University, USA, has devised a scheme that combines plasmonic nanoantennas, which excel at concentrating and enhancing light, with tiny catalytic reactors (PNAS, doi: 10.1073/pnas.1609769113). The result, they suggest, is a “modular” platform that could dramatically expand the horizons for low-temperature, light-driven catalysis across a range of industrial processes.
Energy-intensive processes
Catalysts involving metal nanoparticles are central to industrial chemistry, to speed up reaction times in the manufacture of plastics and other materials. But these catalysts tend to require large energy inputs to do their work; the U.S. Energy Information Agency, for example, has estimated that the production of plastic resin alone consumes almost a quadrillion Btu of energy a year.
One way to ratchet down that energy bill considerably would be if sunlight could be harvested and concentrated to drive catalysis. An excellent way to do so would be to harness surface plasmon resonance—the confinement of incident light into subwavelength oscillations of free electrons that happens at metal-dielectric interfaces. With the right geometry, certain metal nanoparticles, by squeezing light into sub-diffraction volumes, can dramatically concentrate incident optical energy (see “Plasmonic Nanoantennas,” OPN, June 2015).
In the view of many nanotechnologists, plasmonic concentration could be just the ticket to harness and focus the sun’s energy to drive industrial catalysis. But there are two fundamental stumbling blocks. First, metals that are good at catalysis, such as palladium and platinum, tend to be rather poor optical absorbers. And, second, metals that excel at optical absorption and plasmonic enhancement, such as gold, silver and aluminum, are not good catalysts.
Hot carriers
To overcome this catch-22, the Rice scientists, led by OSA Fellow Naomi Halas, attempted to combine the best of both types of metals. They began with 100-nm-diameter crystals of aluminum, and, after slightly oxidizing the crystals, treated them with a palladium salt. The result was a collection of aluminum nanoparticles—excellent plasmonic antennas—that were decorated with “islands” of palladium, a highly efficient catalytic reactor when heated to high temperatures.
In principle, when light is shone on the hybrid antenna-reactor nanoparticles, the subwavelength plasmonic enhancement enabled by the aluminum should boost the local temperature (or, more precisely, the energy available in “hot carrier” electrons) significantly. That, in turn, should create a threshold of energy sufficient to let the palladium islands do their catalytic work.
The researchers tested the scheme in a common process that involves reacting acetylene with hydrogen to produce ethylene, a chemical feedstock for creating the world’s most common plastic, polyethylene. They found that the light-driven approach (using low-power laser sources rather than incident sunlight) was able to churn out ethylene at a 40-to-1 ratio relative to an unwanted by-product, ethane—significantly better selectivity for ethylene than in the thermal catalysis schemes now commonly in use.
Modular approach
One of the exciting aspects of the antenna-reactor design, in the view of the research team, is that the concept “is a highly modular one.” The study notes that, by changing the composition or size of the particles that constitute the plasmonic antennas or the composition of the metal reactor islands, the combination of photocatalytic wavelength and surface chemistry can be finely tuned, allowing the approach to plug into a range of specific reactions and pathways. The result, the study concludes “opens a new door for the development of precise, ultimately predictive control of catalytic chemistry using light.”
