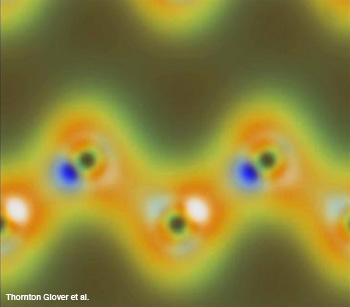
A simulation of the density of charges in the diamond’s outer shell electrons react when intense X-rays and optical waves interact nonlinearly. The carbon appears as dark spots revealed by diffracted X-rays and the peaks of some of the bonds between them show as white and blue spots induced by the polarized optical pulse.
A group of researchers mixed X-rays from a free electron laser and near-IR light from an optical laser to probe how the light polarizes the outer shell electrons of atoms in single-crystal diamond. This proof-of-concept work by Thornton Glover at Berkeley National Lab (U.S.A.) and an international group of scientists could be developed as a tool to better study how light interacts with matter (Nature 488, 603).
Chemical bonds between atoms form among the outer shell electrons. Light interacting with these electrons can form or break bonds. Because the changes occur very quickly and on a size scale of individual atoms, we have not been able to directly examine the dynamics of these interactions.
The scientists used a nonlinear interaction of intense light and X-ray pulses called sum frequency generation (SFG) to probe diamond. Glover's team chose diamond because its structure and electronic properties are well known. Because the X-rays are much more energetic than the IR light, this could also be considered an optically-modulated X-ray experiment. Similar to standard X-ray diffraction, data from inelastically scattered sum-frequency X-rays provides information about the charge density in the electrons. Whereas X-ray diffraction probes all the electrons in an atom equally, the IR light alters the polarization of the valence electrons in a way that can be detected in the scattered X-rays from the SFG radiation.
The technique was proposed in the early 1970s, but X-ray sources were not intense enough to provide fast pulses to create nonlinear interactions. Now, X-rays from the Linac Coherent Light Source (a free electron laser at the SLAC National Accelerator Lab (U.S.A.)) are intense enough, and can be coordinated with picosecond-long pulses from the optical laser to produce SFG. While this experiment used IR light, the method can work for a wide variety of wavelengths.
Further development of this technology could allow us to watch as light triggers atoms to make or break chemical bonds.
