Feature
Perovskite Photovoltaics: The Road Ahead
In terms of efficiency, perovskite solar cells are the fastest-growing solar technology to date, but stability issues still block their widespread commercialization.
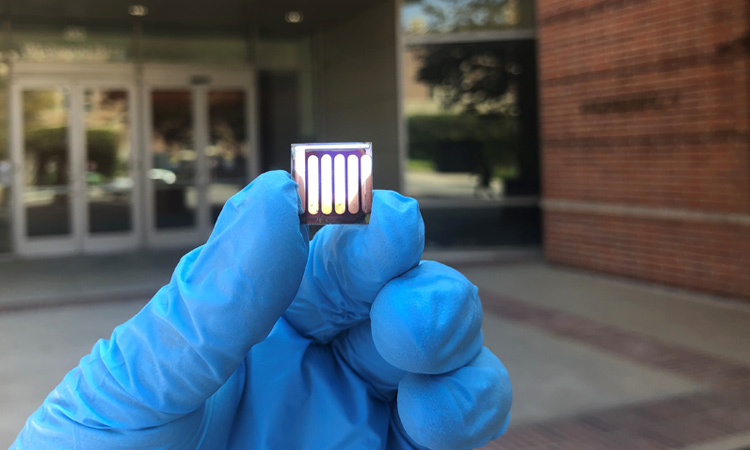 Perovskite solar cell fabricated in Yang Yang’s lab at UCLA. [Jingjing Xue]
Perovskite solar cell fabricated in Yang Yang’s lab at UCLA. [Jingjing Xue]
In 1839, German mineralogist Gustav Rose received a curious delivery from the chief mines inspector of the Russian Empire, August Alexander Kämmerer. It contained a sample of metallic cube-like crystals embedded in a chunk of skarn—a type of rock altered by hot, chemically active fluids—from the Southern Ural Mountains.
…Log in or become a member to view the full text of this article.
This article may be available for purchase via the search at Optica Publishing Group.
Optica Members get the full text of Optics & Photonics News, plus a variety of other member benefits.
