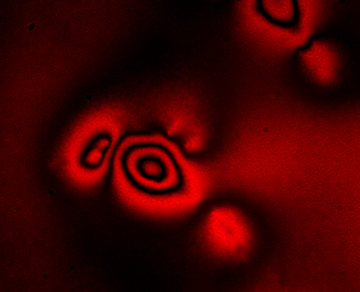
 Elastic resonator interference stress microscopy analyzes interference patterns formed in an ultra-soft deformable optical micro-cavity, to create images of the forces that living cells apply as they grow, divide and migrate—such as this interference pattern generated by a human embryonic kidney cell.
Elastic resonator interference stress microscopy analyzes interference patterns formed in an ultra-soft deformable optical micro-cavity, to create images of the forces that living cells apply as they grow, divide and migrate—such as this interference pattern generated by a human embryonic kidney cell.
Forces lie at the heart of many processes in biology—cell growth, tissue formation, wound healing and the invasion of cancer cells into healthy tissue. The forces exerted by cells vary substantially in magnitude, spatial distribution and temporal evolution.
Recently, there has been strongly increased interest in the role played by vertical forces—the forces that cells exert perpendicular to their substrates. These are important, for example, during the extension of special cellular protrusions called podosomes or during migration of cells through a confined space, in which nonspecific cell–substrate interactions such as friction and pushing become important. Vertical forces are often weak, and existing methods have struggled to resolve them rapidly and non-disruptively.
We have addressed this need by developing a new microscopy method—elastic resonator interference stress microscopy (ERISM)—that allows direct, robust and non-destructive imaging of forces associated with various mechanical cell–substrate interactions.1 Whereas most existing methods use localization microscopy or direct imaging of surface deformations,2 ERISM is inspired by the rainbows of colors that are found on the surface of soap bubbles.
By exploiting a similar optical interference effect in a soft optical microcavity, we were able to detect cell-induced deformations of the cavity with unprecedented sensitivity. This yielded high-resolution maps not only of forces exerted by cells that form firm focal adhesion contacts to the substrate, but also of protein-specific cell–substrate interactions and of the much weaker vertical forces (down to piconewtons) associated with amoeboid-type cell migration through confined environments and with the protrusion of podosomes.
ERISM requires no zero-force reference image, which eliminates the need to detach non-migrating cells after a measurement and enables continuous, long-term measurements of multiple cells on one substrate as well as further investigation of the cells by (for example) immunostaining. As a wide-field imaging method, ERISM determines the local deformation at each point of the image simultaneously and requires only low light intensities, thereby facilitating observation of multiple cells at once without inducing photodamage to the cells. This robustness means that measuring cell forces could soon become a tool in clinical diagnostics, through which doctors could complement existing techniques to assess the invasiveness of cancer.
Researcher
Malte Gather, University of St Andrews, U.K.
References
1. N.M. Kronenberg et al. Nat. Cell Biol. 19, 864 (2017).
2. W.J. Polacheck et al. Nat. Methods 13, 415 (2016).
