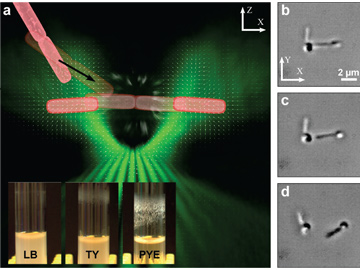
 (a) TOW tweezer principles. Vector field of the trapping beam’s intensity gradient (white arrows), volumetric rendering of the beam near the focus of the objective lens (green), and schematic representation of two rod-shaped bacterial cells entering the trap and being separated (pink). Insert: S. meliloti biofilms in different growth media (LB, lysogeny broth; TY, tryptone yeast extract; PYE, peptone yeast extract). (b to d) Snapshots over time showing TOW optical tweezers dismantling a cluster of S. meliloti cells (see accompanying video).
(a) TOW tweezer principles. Vector field of the trapping beam’s intensity gradient (white arrows), volumetric rendering of the beam near the focus of the objective lens (green), and schematic representation of two rod-shaped bacterial cells entering the trap and being separated (pink). Insert: S. meliloti biofilms in different growth media (LB, lysogeny broth; TY, tryptone yeast extract; PYE, peptone yeast extract). (b to d) Snapshots over time showing TOW optical tweezers dismantling a cluster of S. meliloti cells (see accompanying video).
A better understanding of bacterial biofilms, which contribute to biofouling, corrosion and antimicrobial resistance, could contribute to their management and control.1 Optical tweezers constitute excellent tools for trapping and manipulating bacteria and also for measuring microscopic forces.2-4 Yet conventional single-beam tweezers have difficulty with in-plane observation and manipulation of rod-shaped objects such as bacterial cells, and stretching and separating biological specimens often requires tethering them to trapped beads.
To overcome these hurdles, we have designed “tug-of-war” (TOW) optical tweezers that can aid assessment of cell–cell adhesion, a key factor in biofilm formation.5 These tweezers can stably trap and stretch a rod-shaped bacterium in the observing plane, and can impose a tunable lateral force that pulls apart a cluster of cells without tethering or mechanical movement. The result could be new photonic tools for manipulating biological samples.
The TOW tweezer works by shaping light into two elongated beams with opposite transverse momenta. A tug-of-war duel results, with pulling forces on both sides of the trapped object. The beam size, separation, and propagation direction can be varied at will using a spatial light modulator, allowing interactive control and tuning of the pulling forces.5
We used the system to examine Sinorhizobium meliloti cells, which forms different amounts of biofilm depending on the growth medium. The tweezers were able to trap, stretch, and break apart cells attached to one another in a tryptone yeast medium, whereas clusters formed in a peptone yeast extract medium remained intact even when the power of the trapping beam was increased, an indication of stronger adhesion among the cells.
The demonstration that judiciously shaped light beams can stretch and take apart bacterial clusters offers a simple assay for cellular adhesion, and for quantitatively characterizing forces that bind cells together, shedding light on bacterial aggregation and biofilm formation. The advance may find use in both medical diagnostics and environmental microbiology.
Researchers
Anna Bezryadina, San Francisco State University, Calif., USA (currently at Univ. of California, San Diego, USA)
Daryl Preece, University of Queensland, Brisbane, Australia (currently at Univ. of California, San Diego)
Joseph C. Chen, San Francisco State University
Zhigang Chen, San Francisco State University and Nankai University, Tianjin, China
References
1. H. C. Flemming et al. Nat. Rev. Microbiol. 14, 563 (2016).
2. D. G. Grier. Nature 424, 810 (2003).
3. K. Dholakia and P. Zemánek. Rev. Mod. Phys. 82, 1767 (2010).
4. F. M. Fazal and S. M. Block. Nat. Photon. 5, 318 (2011).
5. A. Bezryadina et al. Light Sci. Appl. 5, e16158 (2016).
