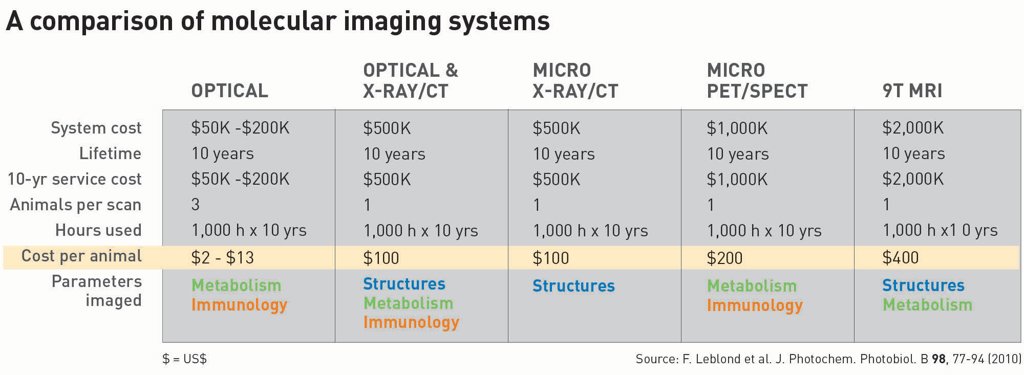
 A new optical imaging technique, Cherenkov-excited luminescence scanned imaging, overlays X-ray tomography of a rat with molecular oxygenation information. [Zhang et al., Opt. Lett. 40, 827 (2015) / Image courtesy of Brian Pogue]
A new optical imaging technique, Cherenkov-excited luminescence scanned imaging, overlays X-ray tomography of a rat with molecular oxygenation information. [Zhang et al., Opt. Lett. 40, 827 (2015) / Image courtesy of Brian Pogue]
Interest in molecular imaging has exploded in the last decade, with a solidly established market for pre-clinical small-animal imaging and with steady growth in the demand for human imaging systems to guide surgery and medical intervention. Competition has heated up as the healthcare industry has increasingly recognized the potential of such technologies not only to improve patient outcomes in an era of personalized medicine, but also, along the way, to create large economic opportunities for whichever systems “win.”
And optical imaging tools are quietly winning that competition. Relative to other imaging methods, particularly traditional radiology tools, molecular imaging in the optical wavelengths can add significant value both in pre-clinical screening of new therapies and in the emergence of guided surgical procedures. While tools like X-ray imaging and liquid-sample screening tests will continue to be a mainstay in diagnostic assessment, direct in vivo optical imaging will carve out an increasingly important role as therapies become more specialized and molecule-specific to the disease—precisely the “sweet spot” of imaging in the optical and near-infrared (NIR) band.
Electromagnetic windows into the body
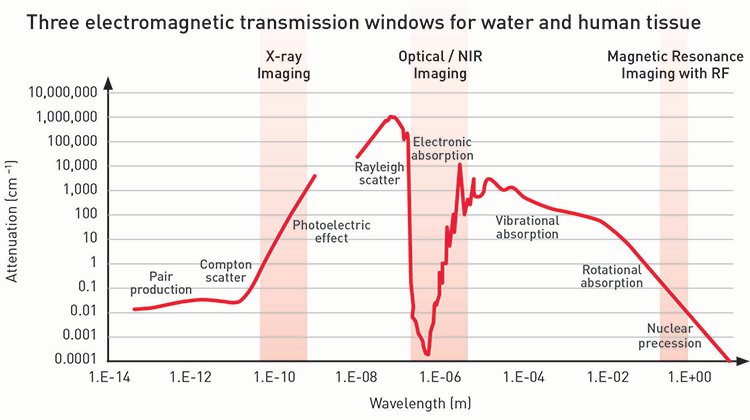
Imaging through tissue requires delivery of active radiation and detection of reflected or transmitted energy—the latter being the most common approach for human and other mammalian tissue. The graph below shows the attenuation of water across the usable electromagnetic spectrum, from high-energy gamma ray wavelengths (around 0.01 nm) to radio frequency wavelengths (around 1 m). Across the spectrum, there are three low-attenuation wavelength ranges, called transmission windows, in which radiation can be transmitted through water. Because our bodies are composed mostly of water, water attenuation is a reasonable surrogate for signals moving in and out of human tissue.
|
|
Of the three transmission windows, the X-ray window is the most commonly used; a majority of humans have received some form of X-ray imaging—for broken bones, cavities, mammograms, or other diagnostics. The radio frequency window is routinely exploited for magnetic resonance imaging (MRI) near 100 MHz for standard 1.5-tesla MRI magnets.
|
The third transmission window is in the optical/NIR wavelength range. It’s actually not a complete transmission window because at optical/NIR wavelengths, there is a dominance of elastic scattering in the body, so tissue appears white when devoid of melanin and blood. Nonetheless, the window finds wide use in reflectance imaging in optical scope systems such as ophthalmoscopes, otoscopes, endoscopes and colonoscopes, which use elastic scattering to create a back-reflected signal to image tissue morphology and color changes. And new methods of molecular in vivo tissue imaging are emerging that take their signal from the attenuation of a specific molecule that has biological significance.
Matching the wavelength band to the molecule
To go beyond the standard structural changes observed with X-ray or radiological imaging tools requires determining which band will provide the molecular information that can drive a diagnosis. Molecular changes manifest themselves through molecular bond differences and, as such, are electronic or vibrational in nature. Such changes have signatures predominantly in the optical and NIR wavelength ranges; therefore, in principle, optical/NIR tools are ideal for measuring these changes. Optically active molecules absorb light into their electronic structure, and the absorption bands are specific to the molecule. Direct molecular signals are rare in the optical range—except for heme, a component of hemoglobin—but can occur in NIR vibrational bands, detected with Raman or other vibrational spectroscopies.
New tools that maximize signal intensity for detection have improved the signal-to-noise ratio of Raman spectroscopy as a molecular signaling tool, and innovations in nano-surface probes with Raman-active molecular probes attached have also emerged, although most of this work is still in the pre-clinical stage. Still, using optical and NIR transitions as a molecular-specific probe looks very promising, because even if exogenously injected, the absorption or luminescent probe signals are specific and can be detected as low-probe concentrations in many in vivo applications. The most significant advances in the field of molecular imaging in vivo have been facilitated by carefully designed molecular probes that are used to sense some specificity within the tissue.
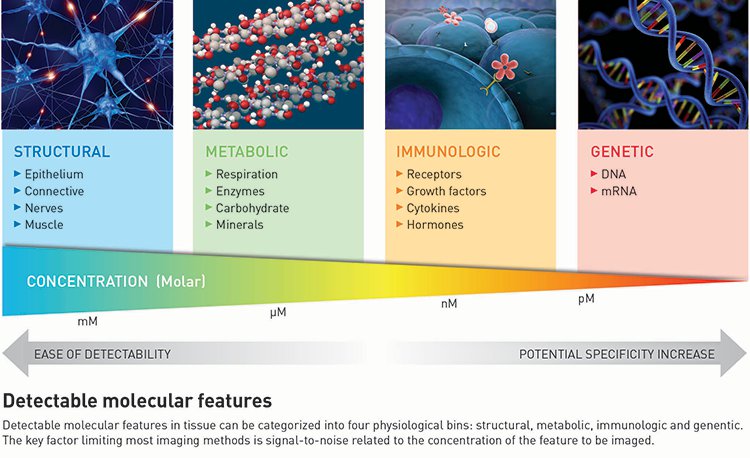
Which molecules matter and at what concentration?
While structural features such as hard tissues, collagen-rich connective tissue, and epithelial cells are easily viewed with imaging tools that detect features in the millimolar (mM) range, gaining more molecular specificity means detecting metabolic and immunologic features in the micromolar to nanomolar (μM to nM) ranges, respectively. Immunological imaging offers perhaps the highest molecular specificity, because receptors, growth factors, cytokines and hormones are expressed directly from the tissue. Yet imaging immunological markers also requires nanomolar-scale sensitivity: even a very high expression rate of 106 receptors per cell corresponds to approximately 100 nM bulk concentration in tissue.
Metabolic markers (enzymes, minerals, pO2, or pH) and immunologic markers (growth factors, cytokines or hormones) can be secreted at concentrations several times higher than surface receptors, which make them easier to detect. However, this secretion means that their location relative to the disease may not be as precise, potentially reducing specificity. Therefore, scientists in the therapeutic and diagnostic worlds are focused on targeting surface receptors or their downstream signals to gain as much specificity as possible from their imaging efforts. But getting molecular tracer reporters to these cellular sites at the innately lower concentrations is challenging, making accurate targeting of receptor density a ripe area for research and development (R&D).
|
|
Scientists face two challenges developing molecular tracer reporters:
Interference from unbound probes. Exogenous probes are usually delivered to a targeted tissue (e.g., a tumor) via the blood stream. But if there are more exogenous probes than receptors on the targeted tissue, the reported signal is dominated by the unbound probes.
Vascular leakage. Most molecular probes are delivered by intravenous injection; therefore, their concentration is inherently affected by vascular leakage patterns that can overwhelm the signal from the small number of bound probes. Techniques to reduce vascular leakage exist, but they are not yet approved for use in humans.
Scientists are also working to develop molecule-activated and quantitative probes that produce a signal only when they bind to the intended receptor. These probes are prime targets for optical imaging because optical switch mechanisms can be inherently electronic, and therefore correspond to the optical/NIR transmission window.
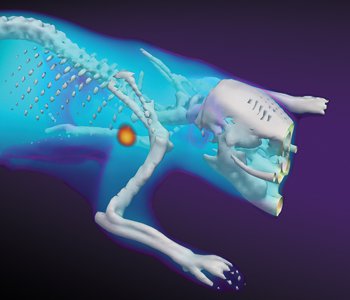
 Molecular imaging requires sensitivity and specificity. Imaging sensitivity to low molecular concentrations determines its use. Optical imaging has a nanomolar-level of sensitivity, parallel to nuclear methods such as PET and SPECT, and has the capability to detect over a dozen different molecular species. [Pogue et al., Adapted from Am. J. Roentgen 195, 321 (2010)]
Molecular imaging requires sensitivity and specificity. Imaging sensitivity to low molecular concentrations determines its use. Optical imaging has a nanomolar-level of sensitivity, parallel to nuclear methods such as PET and SPECT, and has the capability to detect over a dozen different molecular species. [Pogue et al., Adapted from Am. J. Roentgen 195, 321 (2010)]
State of play and market prospects
There are only a few deep-tissue nuclear and optical imaging technologies with sufficient sensitivity to resolve at the µM to nM concentration range. While X-ray, ultrasound, MRI and photo-acoustic techniques could all image 100-µm-scale structural features and conduct metabolic sensing in different scenarios, they do not have the sensitivity to resolve the relevant µM to nM concentration ranges in vivo with high resolution. So, we are currently in a situation where surface imaging with optics can be done with high resolution and high molecular sensitivity, but not deep-tissue imaging at high resolution and high molecular sensitivity.
Nonetheless, optical molecular imaging has carved out niches in small-animal imaging for low-cost, high-throughput pre-clinical/translational research; and in human surface-level diagnostics and surgical guidance. In the balance of this article, we look at the current state of play in those areas, and where the market might be headed.
Small-animal imaging. The market for small-animal imaging systems is well saturated with the current technology, but there is a great need, and an enormous market, for imaging systems that can determine, on a molecular level, whether experimental therapeutics are acting as expected in small-animal models before they enter human “phase 1” clinical trials. These trials are where the amount of money spent grows by a factor of 10 to 100, relative to pre-clinical work; therefore, heading off unsuitable candidate therapeutics has enormous economic benefit to a company.
|
Small-animal imaging is at the forefront of pre-clinical research, using targeted reporter molecules, transfected tumors with reporters, and vascular and cellular probes to predict dosing, response metrics, timing of delivery, and treatment outcomes for potential human therapies. Perhaps no other area of cancer research has been given as much attention as immunologic targeting for altering the intracellular signal transduction and the intercellular trafficking that allow cancer to proliferate and evade treatment.
The direct valuation of optical imaging tools and methodologies is estimated in the billions of U.S. dollars per year, and implications for targeted therapeutics are in the hundreds of billions per year. So, improved small-animal optical molecular-imaging tools could have result in improved cancer outcomes as well as economic success.
The market for existing molecular imaging systems in pre-clinical research matured about a decade ago. Since then, many changes have occurred in the market coalescing around key technological choices. Now, using molecular imaging as an evaluation tool for new therapeutics is becoming a big business. Optical systems are the lowest-cost option by far, with a cost-per-animal in the range of US$2 to US$13. The low cost comes from the use of efficient imaging signals and cameras, and the key ability to image many animals at the same time. Optical systems also offer imaging of metabolic and immunologic changes and targets. Nuclear and MRI platforms have much higher costs-per-animal than their optical counterparts, and micro-computed tomography (CT) has very little to offer in molecular imaging, other than a structural template upon which to overlay optical or nuclear systems. This stark price difference illustrates the value of optical imaging as a low-cost, high-efficiency in vivo screening tool.
Based on the table below, the most cost-effective system by one to two orders of magnitude is optical imaging. It is easy to see why pharmaceutical companies are pursuing pre-clinical optical imaging systems for their R&D, with over half reporting using optical imaging systems for their in vivo work. This has been a trend in the past decade and will likely continue for a long time. While the key elements of a scientific imaging system—including the cooled CCD and fiber optics for light delivery—were invented around 1970, the widespread development and sales of practical versions of each technology occurred in the early 1990s, allowing commercial production systems. These wider uses led to awarding the 2009 Nobel Prize in Physics to the inventors of these two key technologies (Charles Kao, Willard Boyle and George Smith). Now their use as key components of small-animal imaging in the pharmaceutical industry is changing the paradigm of how drug discovery is done.
|
|
Clinical surgical imaging and guidance. New surgical imaging systems have been slow to emerge, yet an explosion has been happening in just the last five years. There has been an important expansion of fluorescence imaging with indocyanine green to view tissue vasculature function and tissue perfusion. This fluorescent probe isn’t technically molecule-specific, but rather provides the baseline for imaging with NIR fluorescence in a surgical setting as a way to enhance real-time visualization of vascular flow. Because the technology has been available to image this tracer, and because it provides unique diagnostic information, surgeons are adopting it in a much wider array of procedures.
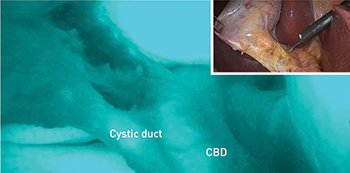 Fluorescent lymphatic mapping during laparoscopic colectomy: Indocyanine-green (ICG) enhanced fluorescence offers real-time high-resolution images of biliary anatomy during laparoscopic gall bladder surgery. When exposed to light in the near-infrared spectrum, ICG fluoresces green, marking the location of the junction between the cystic duct and the common bile duct (CBD).
Fluorescent lymphatic mapping during laparoscopic colectomy: Indocyanine-green (ICG) enhanced fluorescence offers real-time high-resolution images of biliary anatomy during laparoscopic gall bladder surgery. When exposed to light in the near-infrared spectrum, ICG fluoresces green, marking the location of the junction between the cystic duct and the common bile duct (CBD).
Several companies have jumped into the market with NIR-capable systems, and some are now emerging with research-ready multichannel-capable surgical fluorescence systems. Several companies have their systems in clinical trials, and hope to eventually apply for pre-market approval or targeting trials. R&D and funding trends show a clear movement toward molecular imaging for improved surgical guidance.
Some of the more progressive advances have occurred in neurosurgery, such as using protoporphyrin IX fluorescence as a guide for glioma surgery, which was approved in Germany after a 2006 multicenter clinical trial. There’s growing interest in other fluorescent reporter proteins, but definitive clinical trials are still ongoing. Perhaps the best indicator of success is the emergence of companies manufacturing specific fluorescent molecular-probes and the surgical instrumentation companies debuting new multi-wavelength systems.
Despite the economic challenges of developing linked tools and molecular probes, many indicators, including publications, established companies and device sales, seem to be pointing to continued growth in optical molecular imaging to advance surgical guidance.
OSA Fellow Brian W. Pogue is professor of engineering science, physics & astronomy and surgery at Dartmouth College, USA.
References and Resources
-
R.N. Gunn. “Parametric imaging of ligand-receptor binding in PET using a simplified reference region model,” Neuroimage 6, 279 (1997).
-
C. Bremer et al. “Optical-based molecular imaging: contrast agents and potential medical applications,” Eur. Radiol. 13, 231 (2003).
-
K. Sokolov et al. “Optical systems for in vivo molecular imaging of cancer,” Technol. Cancer Res. Treat. 2, 491 (2003).
-
S. Achilefu. “Lighting up tumors with receptor-specific optical molecular probes,” Technol. Cancer Res. Treat. 3, 393 (2004).
-
W. Stummer et al. “Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial,” Lancet Oncol. 7, 392 (2006).
-
T. Barrett et al. “In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies,” Clin. Cancer Res. 13, 6639 (2007).
-
E.D. Agdeppa and M.E. Spilker. “A review of imaging agent development,” AAPS J. 11, 286 (2009).
-
L.P. Garrison Jr. and D.L. Veenstra. “The economic value of innovative treatments over the product life cycle: the case of targeted trastuzumabtherapy for breast cancer,” Value Health 12, 1118 (2009).
-
H. Gong et al. “In vivo imaging of xenograft tumors using an epidermal growth factor receptor-specific affibody molecule labeled with a near-infrared fluorophore,” Neoplasia 12, 139 (2010).
-
F. Leblond et al. “Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications,” J. Photochem. Photobiol. B 98, 77 (2010).
-
J. Comley. “In vivo preclincal imaging: An essential tool in translational research,” Drug Discov. World 11, 58 (2011).
-
D. Delbeke and G.M. Segall. “Status of and trends in nuclear medicine in the United States,” J. Nucl. Med. 52 Suppl 2, 24S-8S (2011).
-
G.M. van Dam et al. “Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results,” Nat. Med. 17, 1315 (2011).
-
K.M. Tichauer et al. “In vivo quantification of tumor receptor binding potential with dual-reporter molecular imaging,” Mol. Imaging Biol. 14, 584 (2012).
-
M. Freebody. “Trends in imaging: Whole-animal imaging for preclincal research,” Biophotonics December (2013).
-
A. Mullard. “Molecular imaging as a de-risking tool: coming into focus?” Nat. Rev. Drug Discov. 12, 251 (2013).
-
L. Boni et al. “Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery,” Surg. Endosc. (2014).
-
K.S. Samkoe et al. “Quantitative in vivo immunohisto-chemistry using a dual-tracer receptor concentration imaging approach,” Cancer Res. 15, 7465 (2014).
-
S. Harmsen et al. “Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging,” Sci. Transl. Med. 7, 271ra7 (2015).
-
J.H. Hubbell and S.M. Seltzer. “Tables of X-ray mass attenuation coefficients,” NISTIR 5632 (2015).
-
M. Jermyn et al. “Intraoperative brain cancer detection with Raman spectroscopy in humans,” Sci. Transl. Med. 7, 274ra19 (2015).
-
T. Namikawa et al. “Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green,” Surg. Today (2015).
-
S. Prahl and S. Jacques. “Optical properties spectra,” Oregon Medical Laser Center, http://omlc.org/spectra/
-
Radioisotopes in Medicine. www.world-nuclear.org/info/non-power-nuclear-applications/radioisotopes/radioisotopes-in-medicine/ (April, 2015).

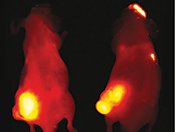 Visible/near-infrared luminescence imaging, the technique used to create this image of protease activation in mouse tumors, is the most commercially dominant system.
Visible/near-infrared luminescence imaging, the technique used to create this image of protease activation in mouse tumors, is the most commercially dominant system. Photo-acoustic imaging, used to create this image of a mouse’s abdominal blood vessels, is the newest and fastest-growing modality.
Photo-acoustic imaging, used to create this image of a mouse’s abdominal blood vessels, is the newest and fastest-growing modality.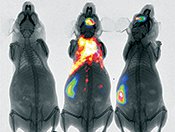 Hybrid modalities for structural and functional imaging, like the combination used to create this image of inflammation-induced myeloperoxidase activity in mice, are the most desirable and the most expensive systems.
Hybrid modalities for structural and functional imaging, like the combination used to create this image of inflammation-induced myeloperoxidase activity in mice, are the most desirable and the most expensive systems.