 An artist’s conception of the ChemCam instrument aboard the Curiosity rover in operation. The ChemCam can be used to determine the chemical composition of the various sedimentary layers of the plant.
An artist’s conception of the ChemCam instrument aboard the Curiosity rover in operation. The ChemCam can be used to determine the chemical composition of the various sedimentary layers of the plant.
When NASA’s rover Curiosity landed successfully on Mars last August, it opened a new chapter in space exploration: the use of laser-based instruments to probe the surface composition of other planets. Otherwise known as the Mars Science Laboratory (MSL), the nearly one-ton rover was designed to be a laboratory on wheels, capable of doing sophisticated analyses on rough terrain on the red planet.
The Curiosity mission is expected to reveal a rich portrait of Martian history, particularly since the decision was made to land the vehicle in the 150-km Gale crater, which was formed about 3.6 billion years ago during a humid period on Mars. With the tallest stack of accessible sedimentary rock layers in the solar system (taller than the Grand Canyon is deep), the crater may contain details of a climate change from the wetter, warmer past to the cold, dormant conditions today.
A laser in the Curiosity toolbox is “zapping” samples along the rover’s path to determine their composition. One of MSL’s overarching goals is to determine if the area was or is potentially habitable—that is, whether or not it could have sustained liquid water in the presence of other chemicals necessary for life, such as carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur.
 NASA’s Curiosity rover landed in the Gale crater. The rover landed well within its targeted landing ellipse (20 km x 6.4 km), outlined in blue. Mount Sharp with an elevation of about 5.5 km above the floor of the crater is located in the center.
NASA’s Curiosity rover landed in the Gale crater. The rover landed well within its targeted landing ellipse (20 km x 6.4 km), outlined in blue. Mount Sharp with an elevation of about 5.5 km above the floor of the crater is located in the center.
The laser is part of an instrument called ChemCam (chemistry and camera), which analyzes samples through a process called laser-induced breakdown spectroscopy (LIBS). ChemCam includes a remote LIBS spectrometer and a remote micro-imager (RMI).
ChemCam’s mission is to perform:
-
Rapid rock classification: ChemCam will compare LIBS spectra to a Mars spectral library, allowing researchers to find unique signatures that will identify the types of rocks, thereby indicating which samples are worth studying with more time-intensive Curiosity instruments.
-
Quantitative composition: This will be done for major, minor and trace elements using multiple analysis points, along with a spectral training set.
-
Depth profiling: ChemCam can remove dust and drill through layers of rock and soil by repeatedly directing laser pulses on a specific location. These data could provide important clues to climate and weather conditions over the history of the sample. The lasers can profile up to one millimeter in rock and several millimeters in soil.
-
Context imaging: RMI provides high-resolution images for analyzing LIBS targets. This is important for investigating the 350- to 550-μm LIBS observation pits and mineral grain sizes.
-
Passive spectroscopy: ChemCam’s spectrometers have a passive setting for collecting data on, for example, the iron content of samples. This capability extends beyond the 7-m limit of the laser interaction.
A new era for space exploration: LIBS on Mars
LIBS was born in 1962 when Brech and Cross reported laser-induced spark emission. They focused a ruby laser beam onto metallic and non-metallic solid materials to generate vapors that were subsequently excited with an electric spark to generate emission spark spectra.
 NASA’s Curiosity rover used the Mars Hand Lens Imager (MAHLI) to capture 55 high-resolution images, which were stitched together to create this full-color self-portrait. The ChemCam, two mast cameras and four navigation cameras can be seen at the top of the remote sensing mast.
NASA’s Curiosity rover used the Mars Hand Lens Imager (MAHLI) to capture 55 high-resolution images, which were stitched together to create this full-color self-portrait. The ChemCam, two mast cameras and four navigation cameras can be seen at the top of the remote sensing mast.
|
LIBS consists of focusing a pulsed laser onto a target to create a plasma plume and analyzing the optical spectra made up of emission lines generated as the plume decays. The unifying feature of all LIBS experiments is the formation of a laser-generated plasma plume from the target, which can be a solid, a liquid or a gas. The fundamental processes that create the plasma are very complex and depend on the characteristics of the target and its surrounding atmosphere, as well as the features of the laser.
As a spectrochemical analytical technique, LIBS can provide the elemental composition of a sample in a solid, liquid or gaseous phase without sample preparation. It relies on the spectral analysis of atomic, ionic and potentially molecular bands emitted by the plasma plume initiated above the surface of the sample. Due to its versatility and simplicity, LIBS has been used for a wide range of analytical applications, including simultaneous multi-elemental analyses of metals and biological samples.
LIBS experiments are usually conducted under standard Earth atmospheric conditions. About 20 years ago, Blacic et al. suggested using LIBS for the remote analysis of planetary surfaces. This year, on the 50th anniversary of the technique, LIBS was used on the red planet, more than a hundred million kilometers away from where it was first developed. LIBS experiments on Mars are particularly challenging because researchers have to deal with atmospheric conditions significantly different than those in a laboratory on Earth, including:
-
An atmospheric pressure of 7 Torr (100 times lower than that on Earth).
-
Surface temperatures that range from −125 °C during polar night to 27 °C at the equator during midday.
-
An atmosphere composed of 95.9 percent carbon dioxide, 1.9 percent nitrogen, 1.9 percent argon, 0.14 percent oxygen and 0.06 percent carbon monoxide.
-
A surface typically covered with dust or other debris.
-
High surface winds of 10 m/s, with gusts up to 40 m/s.
Habitability on Mars
Mars is considered potentially habitable because it has abundant water and a thin atmosphere; billions of years ago it had a climate more similar to Earth. Our study of habitability on Mars is buoyed by an explosion of findings related to microscopic organisms on Earth that thrive in extreme environments—including those characterized by high pressure, heat or radiation—indicating that life might have a chance on the surface of Mars, which is subject to relatively high radiation doses compared to Earth. Some of these terrestrial “extremophiles” obtain their energy from surprising sources such as the oxidation or reduction of manganese on rock surfaces.
The search for evidence of past or present life on Mars requires a better and broader understanding of the geochemistry of Martian rocks and soils and a characterization of their biological potential or biosignatures. While a number of scientific approaches have been explored to investigate the likelihood of biosignatures on planets, ChemCam provides an almost complete elemental composition of selected targets, including elements important to life on Earth.
Recent investigations have demonstrated that LIBS can be used in quantitative molecular analysis to characterize complex biosamples, including organic carbon in soils, and to obtain hydrogen composition of high-molecular-weight proteins.
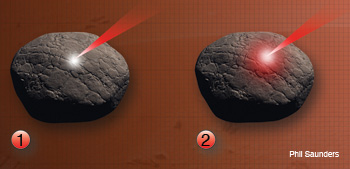 Laser-Induced Breakdown Spectroscopy: The entire LIBS process lasts up to tens of microseconds. (1.) Laser/absorbtion: A pulsed laser is focused on a target (rock). An irradiance threshold of 1 GW/cm2 is needed to obtain a plasma representative of the target composition. Part of the laser’s energy is absorbed by the target and generates ions and free electrons, which gain kinetic energy. (2.) Heating: The free electrons collide with the lattice phonons on the rock’s surface, leading to avalanche ionization and thermal energy (red spot on rock) in the target.
Laser-Induced Breakdown Spectroscopy: The entire LIBS process lasts up to tens of microseconds. (1.) Laser/absorbtion: A pulsed laser is focused on a target (rock). An irradiance threshold of 1 GW/cm2 is needed to obtain a plasma representative of the target composition. Part of the laser’s energy is absorbed by the target and generates ions and free electrons, which gain kinetic energy. (2.) Heating: The free electrons collide with the lattice phonons on the rock’s surface, leading to avalanche ionization and thermal energy (red spot on rock) in the target.
 (3.) Melting/evaporation: A small portion of the target rich in free electrons, ions and neutrons is vaporized and ablated (white cloud and divot); the ablated region expands at a velocity faster than the speed of sound and generates a shock wave. (4.) Plasma: As the vapor expands, it continues to absorb optical energy from the laser and becomes a luminous plasma plume (white cloud) with temperatures that can reach 104 K.
(3.) Melting/evaporation: A small portion of the target rich in free electrons, ions and neutrons is vaporized and ablated (white cloud and divot); the ablated region expands at a velocity faster than the speed of sound and generates a shock wave. (4.) Plasma: As the vapor expands, it continues to absorb optical energy from the laser and becomes a luminous plasma plume (white cloud) with temperatures that can reach 104 K.
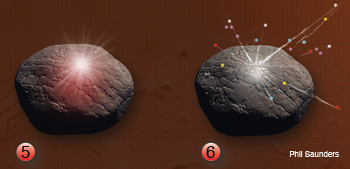 (5.) Emission: The laser pulse ends. The plasma plume collides with the surrounding species and begins to cool, emitting radiation through emission and recombination processes that can be collected to analyze the target (white cloud). (6.) Species formation: The radiative and non-radiative (post-plasma) processes may yield high-density neutral species, clusters of nanometer and micrometer sized particles (multicolored dots).
(5.) Emission: The laser pulse ends. The plasma plume collides with the surrounding species and begins to cool, emitting radiation through emission and recombination processes that can be collected to analyze the target (white cloud). (6.) Species formation: The radiative and non-radiative (post-plasma) processes may yield high-density neutral species, clusters of nanometer and micrometer sized particles (multicolored dots).
ChemCam: A high-res camera and spectrometer
To meet the MSL objectives, the ChemCam laser had to satisfy a number of challenging requirements: an output intensity of more than 10 MW/mm2 during each pulse, environmental conditions characterized by a minimum of 670 diurnal thermal cycles for the nominal mission duration, and compactness and weight not to exceed 140 cm3 and 600 g.
The ChemCam laser consists of a diode-pumped, rubidium titanium phosphate Pockels cell Q-switched, neodymium-doped potassium-gadolinium tungstate (Nd:KGW) linear oscillator and two amplifiers. The oscillator and amplifiers are pumped individually by a 700-W diode stack. Nd:KGW was selected as the operating medium because it provides small absorption variations over a broad temperature range. It yields a stable power output without cooling, even in an environment characterized by large daily temperature variations.
The ChemCam laser operates at temperatures between −30 °C and 30 °C and generates about 30 mJ of 1,067-nm radiation in about 5-ns long pulses with an M2 of less than 1.6 over most of the operating thermal range, a beam diameter of 2.5 to 3.5 mm, a pointing stability and a pointing direction of less than 0.3 mrad, and a shot-to-shot energy stability with less than 2 percent root mean square variation. The oscillator provides an output energy of about 10 mJ. The two amplifiers give a three-fold gain per pulse.
The oscillator and amplifiers are housed inside a hermetically sealed titanium box filled with dry air and a getter. This setup protects the laser from environmental conditions, reducing the risk of self-contamination and absorbing potential pollutants. The laser was tested under the expected conditions on Mars—particularly in terms of thermal, radiative and mechanical constraints—before its integration with the rest of the ChemCam instruments. It was space qualified by Thales Optronique in France; France’s Centre National des Etudes Spatiales; and Los Alamos National Laboratory and the Jet Propulsion Laboratory in the United States. To measure the emission lines of LIBS signals, ChemCam uses three spectrometers that operate in UV, visible and visible-to-near-IR spectral regions.
A key feature of ChemCam is its ability to use LIBS to detect nearly all chemical elements—including light elements that previous Mars rovers couldn’t measure: hydrogen, lithium, beryllium, boron, carbon, nitrogen and oxygen. Before the launch of Curiosity, researchers demonstrated that the ChemCam laser could quantify most major elements in geological samples to ±10 percent relative accuracy. The ChemCam team is working to realize this accuracy on Mars samples. In view of the LIBS spectra being generated by ChemCam, current data indicate that the laser is performing to a level that satisfies all the requirements of the mission and promises to zap more than one million laser pulses on Martian rocks and soils.
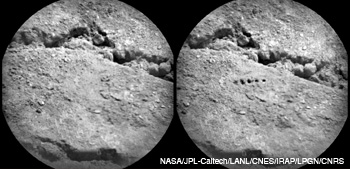 On day 19 of the mission, Curiosity sent RMI images of the “Beechey” target (a sample of Martian soil) taken before (left) and after (right) laser ablation. The image on the right shows five laser-ablated regions generated by using 50 laser pulses with an irradiance of about 30 MW/mm2 in each pulse.
On day 19 of the mission, Curiosity sent RMI images of the “Beechey” target (a sample of Martian soil) taken before (left) and after (right) laser ablation. The image on the right shows five laser-ablated regions generated by using 50 laser pulses with an irradiance of about 30 MW/mm2 in each pulse.
The ChemCam also houses the remote micro-imager—a high-resolution camera that it inherited from the European Space Agency project Rosetta. The RMI provides geomorphology and textural data of Martian rocks and soils as well as context images of the LIBS targets. With a field of view of 20 mrad and a capability to identify a 1-mm feature from 10 m, the RMI can also visualize features within the 0.35 to 0.55 mm diameter ablated regions before and after laser zapping. Five ablated regions, enlarged further by the laser shock wave, are clearly visible in the image on the facing page.
In the figure below, we show the first LIBS spectrum of a Martian rock named “Coronation.” This spectrum, sent to Earth on 19 August 2012, represents the average of 30 LIBS spectra measured at a single location. For this specific measurement, the hydrogen peak was only present on the first laser shot, indicating that hydrogen was only present on the top surface of the rock.
|
|
Machine learning for LIBS signal analysis
One of the goals of ChemCam is to analyze LIBS signals coupled to RMI images. These data can be used to rapidly extract compositional information of Martian rock and soil samples, which can then be classified using a database of well-characterized samples. The classifications are then used to determine if a more in-depth analysis with other Curiosity instruments is appropriate. The amount of data generated with LIBS is large compared to the number of elements contributing to its signal.
As a result, it is necessary to reduce the dimensionality of the data before a classification algorithm can be applied. To suppress unimportant data and highlight useful information, ChemCam researchers have explored a number of dimensionality reduction techniques based on multivariate analyses. These include principal component analysis, independent component analysis, partial least squares, soft independent modeling of class analogy and non-linear Sammon’s map projection. These complementary methods provide physical meaning to the independent components retrieved and a fast and easy way to visually demonstrate the accurate classification of zapped Martian rocks and soils.
What’s next?
As of November 2012, ChemCam has directed over 12,000 laser shots at rocks and soils on Mars, and the interpretation of the LIBS analyses of Mars are under way. Time will tell how useful LIBS is in helping Curiosity in its search of habitable environments on our neighboring planet. With good fortune, ChemCam’s team will have a very rewarding future. Pulsed lasers have entered an era where they too can contribute to answering one of the oldest question humans have ever asked: Are we alone in the universe?
N. Melikechi is with Delaware State University, U.S.A.; R. Wiens is with Los Alamos National Laboratory, U.S.A.; H. Newsom is with the University of New Mexico, U.S.A.; and S. Maurice is with the University Paul Sabatier—CNRS, France. All co-authors are members of the Mars Science Laboratory scientific team.

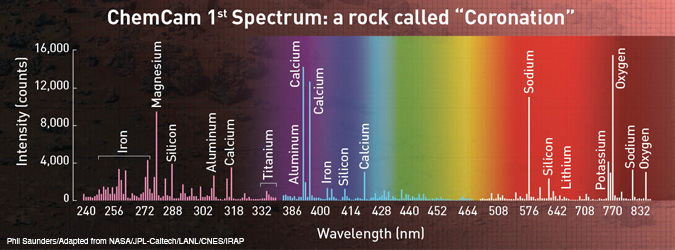 ChemCam’s first Martian laser-induced breakdown spectrum was obtained remotely on day 13 of the MSL mission. It is the result of a laser hitting “Coronation,” a fist-sized rock located 2.6 m away from the ChemCam.
ChemCam’s first Martian laser-induced breakdown spectrum was obtained remotely on day 13 of the MSL mission. It is the result of a laser hitting “Coronation,” a fist-sized rock located 2.6 m away from the ChemCam.