Arthur Ashkin ushered in the field of optical manipulation more than 40 years ago, when he used a new light source called the laser to decouple the optical and thermal forces associated with light so that radiation pressure could be harnessed without being drowned out by heat effects. In 1986, he developed the optical tweezer technique that uses laser light to hold tiny objects in a stable position by surrounding it with equal force in three dimensions.
Since then, work in this area has mushroomed, and researchers in physics, biology and many other fields have developed numerous methods for confining and controlling particles, working toward developing more practical methods to hold and manipulate tiny targets such as single cells or particles.
Now, scientists are finding ways to manipulate even smaller-scale objects, including nanometer-scale proteins and DNA, by creating new techniques such as holographic optical trapping and fiber-optic tweezers, and using them to better understand stem-cell migration and brain behavior.
Optical tools for manipulating neurons
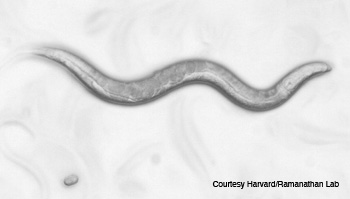
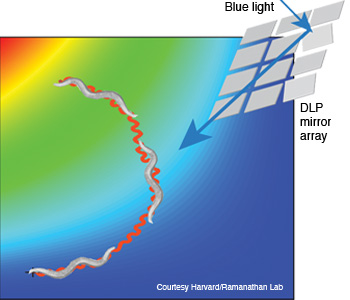 Manipulating the brain with light: The C. elegans nematode (shown with an egg, top) responded to precise stimulation of the AIY interneuron pair with blue 480 nm light (right), enabling researchers to direct its movement.
Manipulating the brain with light: The C. elegans nematode (shown with an egg, top) responded to precise stimulation of the AIY interneuron pair with blue 480 nm light (right), enabling researchers to direct its movement.
When it comes to understanding how the brain works, researchers who study electrical activity patterns need a way to operate in the world of the very small, very quickly. On that topic, at Harvard University, researcher Sharad Ramanathan and colleagues definitely know how to isolate the proverbial needle in a haystack—or at least neurons within the brain of a tiny, transparent worm. The team used optogenetics, non-contact microscopy techniques and precisely targeted lasers to control and manipulate the brain and behavior of the 1-mm-long Caenorhabditis elegans nematode.
Figuring out the dynamics of neural activity is generally very complicated, since it involves trying to pinpoint which behaviors are linked to specific synapses, or structures through which nerve cells (neurons) pass electrical or chemical messages to one another. The human brain contains hundreds of trillions of synapses. Fortunately, the brain of the nematode is much more manageable, with a small number of neurons (302) and synapses (7,000).
The team first deduced the single interneuron pair that would evoke chemotactic behavior—which enables organisms to locate and track food. However, to optically stimulate a single 5- to 10-µm neuron in a tiny animal that moves and changes direction at 150 ± 50 µm s–1 required a custom real-time system that could image, identify and illuminate the exact spot within 25 ms.
The closed-loop excitation system they built sent 480- and 540-nm LED light off a digital-light-processing mirror array, through long-pass and band-pass filters and an illuminated stage into a video camera. At the same time, a 540-nm imaging source was directed onto the stage where the nematode was stimulated by the light gradient and imaged by an electron-multiplying CCD camera. In other words, the optical system allowed the researchers to both manipulate the neurons of interest and track behavior at the same time.
|
“We used a known light-sensitive protein to control the animal’s behavior,” said postdoctoral researcher Askin Kocabas at the FAS Center for Systems Biology. “While the important contribution to biology is identifying and manipulating the interneuron that controls crucial locomotory behavior, the novel optical system provides a framework that allows others in the scientific community to duplicate the work of controlling the complex nervous system.”
The tender trap
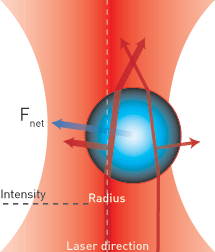
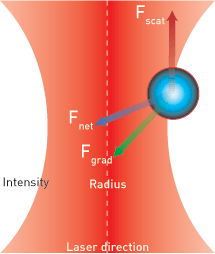
Another method of controlling small things using light is to use an optical trap, also known as optical tweezers. Techniques to optically trap, control and manipulate microscopic objects have received much attention recently in the scientific community, particularly for tomographic imaging and microfluidic applications, says Samar Mohanty, assistant professor of physics at the University of Texas at Arlington. A key feature of this technology is that it allows researchers to control and rotate single cells without making physical contact with them and thus disrupting their structure or functioning.
Mohanty and colleagues recently reported the development of a fiber-optic spanner that can trap and rotate smooth human muscle cells while overcoming the short working distance normally associated with high-numerical-aperture microscope objectives.
The spanner holds a sample between two 20-mW counterpropagating beams sent down single-mode, 8-µm–diameter optical fibers offset transversely by 10 µm. The first fiber carries a divergent, near-infrared beam of 980 nm in continuous wave mode, while the second is coupled to a butterfly laser diode at 975 nm; slightly different wavelengths prevent the construction of standing waves.
The technique works via the scattering force to trap objects axially between the two beams and the gradient force in the transverse plane. The scattering forces from the two beams also set up a torque about the center of the object to rotate it about the axis normal to the fibers. The object can be simultaneously translated and rotated by varying the laser power of one fiber-optic arm. Samples are not limited by their structural and optical properties.
“Because the fiber-optic spanner can be easily integrated onto a microfluidic platform,” says Mohanty, “the configuration is ideal for lab-on-a-chip devices.”
Mohanty and colleagues further tested the applicability of the spanner by combining it with quantitative phase microscopy to tomographically image normal and diseased cells. This approach will enable researchers to test several basic hypotheses and postulates, including cell division and other cellular functions when cells undergo rotation.
“We are also interested in monitoring the change in the rotation speed of cells when they transform to a diseased state and whether we can use it for diagnosis,” says Mohanty.
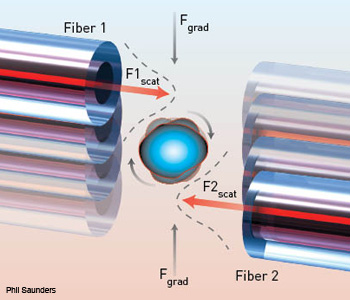 Fiber-optic spanner: The basic setup is comprised of two 125-µm–diameter fibers transversely offset by 20 mW in each arm. The fibers are able to trap and smoothly rotate human smooth muscle cells between them.
Fiber-optic spanner: The basic setup is comprised of two 125-µm–diameter fibers transversely offset by 20 mW in each arm. The fibers are able to trap and smoothly rotate human smooth muscle cells between them.
Biomaterial scaffolds
Optical tweezers are considered a “field gradient” method of optically manipulating cells and other tiny particles because they trap objects between opposing fields. Non-optical field-gradient techniques include magnetic tweezers and dielectrophoretic traps.
All of these methods require a liquid medium in a two-dimensional environment—which isn’t the ideal condition for most cells. For that reason, scientists are now working to develop methods for manipulating cells within carefully designed biomaterial “scaffolds” that mimic their natural surroundings.
The scaffolding method is particularly promising for studying cell migration, which refers to the orchestrated movement of a cell in a specific direction; this is a natural process that is essential in wound healing, tissue development and the formation of embryos.
Professor of biomedical engineering Jennifer Elisseeff and colleagues at Johns Hopkins University devised a strategy in which they manipulated human stem cells in synthetic hydrogels. The cells had been transfected with a protein called Rac that plays a key role in cell migration and that responds specifically to 458-nm light. The team used a laser to activate the protein and thus induce cell migration in the direction of the light.
The laser repeatedly activated the cell in a subcellular spot 10 μm in diameter for 30-s periods at 80-s intervals. The team studied how the hydrogel matrix influenced the activity of Rac in stem cells using fluorescence resonance energy transfer (FRET) imaging—a technique that measures the distance-dependent energy transfer between molecules, which can be correlated with protein activity.
|
They found that manipulating the stiffness of the gel matrix, or “scaffold,” influenced cell migration, with more stiffness resulting in an increased rate of movement. The gels included a photodegradable material that allowed the researchers to then use light to create channels within it. The cells migrated immediately into the open spaces, whereas movement was much slower in the bulk gel.
This work will help researchers to better understand—and mimic—the natural cellular processes that enable tissue regeneration and repair and how they are influenced by the environment surrounding cells.
“This light tool brought us a step closer to controlling the cells in an active way instead of passively,” says first co-author Qiongyu Guo.
Nanotweezers
Conventional optical tweezers are useful for manipulating microscale objects, but they can’t exert enough force to manipulate dielectric materials smaller than about 100 nm. In the Rayleigh regime, the object to be carefully manipulated is much smaller than the wavelength of light used to control it—akin to handling tiny screws while wearing giant mittens. Furthermore, says Robert Hart, president and CEO of Optofluidics (Philadelphia, Pa., U.S.A.), the trapping force scales with the third power of the particle’s diameter.
“Something that’s 10 nm across is a thousand times more difficult to control than a 100-nm object,” says Hart. “That’s why it’s been so hard to trap such small objects. Keeping things in place gets harder the smaller they are. Small things in liquid, even if they’re inanimate, are always careening around in classic Brownian motion when you zoom in on them.”
Recently, researchers have developed several near-field optical trapping techniques, including plasmonic optical tweezers, slot waveguides and whispering-gallery-mode resonators that can provide higher trapping stiffness, but they are limited in their trap-and-release control of smaller objects due to their use of extreme electromagnetic fields and a resulting heat.
Optofluidics, a spin-off company based on technology developed at Cornell University (Ithaca, N.Y., U.S.A.), is commercializing a new form of photonic crystal “nanotweezer” that can trap and release proteins associated with specific diseases, quantum dots and polymer particles with a temperature rise of less than approximately 0.3 K—well below the point where fluid mechanical effects prevent trapping and where biological targets would be damaged by heat.
The “Molecular NanoTweezer” uses the evanescent field of light traveling in waveguides rather than free-space optics. Evanescent light forms along a boundary from one transmission medium to another—for example, from air to water, or, in this case, from water to the silicon nitride of a waveguide. The propagating portion of light is visible and moves along the waveguide. The evanescent portion is an invisible “aura” that hugs the exterior of the waveguide.
“The waveguide looks dark on the outside,” says Hart, “but if you could move your eye 100 nm away, you could detect it.” He describes the effect as very weird. “When you are dealing with diffraction-limited science, it doesn’t make sense in our world, but quantum effects don’t work conventionally.”
This evanescent field, which is an effect of quantum mechanics, decays exponentially as you move a couple hundred nanometers normal to the surface of the waveguide. Typically an evanescent field is weak and doesn’t have much influence. But as a waveguide gets smaller, the relative amount of light in the evanescent field increases. The waveguides used in the NanoTweezer from Optofluidics are a couple of hundred nanometers tall, so the evanescent field extends as much as 0.5 µm from the waveguide.
“A typical tweezer focuses a traditional laser beam into a focal point,” says Optofluidics CEO Bernardo Cordovez. “To obtain a smaller focal point and trap smaller objects, you need a stronger optical gradient. We use the evanescent field itself, which works like a gradient.”
Because the gradient is sub-diffraction–sized, the amount of precision available to trap an object is much greater than it is with traditional optical tweezers. The stiffness of an optical trap is a traditional force spectroscopy measurement—typically measured in terms of force/displacement (e.g., piconewtons/nm). The power-normalized stiffness of the nanotweezer optical trap in the X and Y direction are approximately 0.14 pN nm–1 W–1 and 0.09 pN nm–1 W–1, respectively.
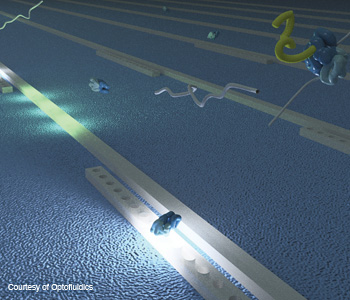 3-D nanotweezers: A three-dimensional rendering of the Optofluidics “NanoTweezer” technology depicts particles flowing over optical waveguides. When stimulated by infrared light, the particles are attracted to the evanescent field and reversibly immobilized until the light is turned off, like a tractor beam.
3-D nanotweezers: A three-dimensional rendering of the Optofluidics “NanoTweezer” technology depicts particles flowing over optical waveguides. When stimulated by infrared light, the particles are attracted to the evanescent field and reversibly immobilized until the light is turned off, like a tractor beam.
The NanoTweezer waveguide also incorporates a series of resonators that act as special mirrors to trap light. When the right wavelength travels down the waveguide and comes in contact with these features, the light constructively interferes, amplifying the intensity of the evanescent field. This greatly increases the amount of force that can be applied by the trap.
This small and powerful combination is what enables NanoTweezer technology to get around the classic diffraction limit. In operation, when particles flow over the illuminated waveguides, they interact with the evanescent field and are pulled down and reversibly immobilized on it. The trapped particle remains immobilized until the light is turned off, like a tractor beam, says Hart.
This optical gradient can trap a whole new class of previously inaccessible nanoscale objects that are particularly valuable to bioscience, including those smaller than 100 nm (the limitation of traditional optical tweezers), such as bacteria, viruses, DNA, molecules and proteins.
The problem with heating that affects other optical microscopy trapping techniques is addressed by the material used in the NanoTweezer waveguides.
“Silicon nitride has a low optical absorption at the infrared wavelength of the laser we use (1,064 nm),” says Cordovez. “This means that the amount of heating is very small.”
The smallest object that can be trapped is an individual protein, a molecule on the order of 5 nm in size, or 45 kilodaltons (KDa), where KDa denotes the mass of a molecule. The NanoTweezer system can be affixed to existing microscope stage mounts. The early-stage company is in the process of launching their first product and plans to begin marketing its NanoTweezer soon to college laboratories.
Getting a grip
The past four-plus decades have seen optical manipulation blossom far beyond simple optical traps. This explosion of work is unlikely to slow down as more and more physicists become interdisciplinary scientists and move into applied areas in biophysics and chemistry. Technology development continues as well.
Recently, some researchers have been working to scale up the optical tweezer concept, with at least one report of a real version of “tractor beams” (see related article). A world of possibilities for optical manipulation—whether nano, micro or macro—is now within our grasp.
Valerie C. Coffey is a freelance science and technology writer and editor based in Boxborough, Mass., U.S.A.
References and Resources
-
C. Webb and J. Jones, ed. “Handbook of Laser Technology and Applications,” Institute of Physics, London (2004).
-
B. Black and S. Mohanty, “Fiber-optic spanner,” Opt. Lett. 37, 5030-5032 (2012).
-
Y.-F. Chen et al. “Controlled photonic manipulation of proteins and other nanomaterials,” Nano Lett. 12, 1633 (2012).
-
Q. Guo et al. “Light activated cell migration in synthetic extracellular matrices,” Biomaterials 33, 8040 (2012).
-
A. Kocabas et al. “Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behavior,” Nature 490, 273 (2012).
-
See “Molecular NanoTweezer” video.

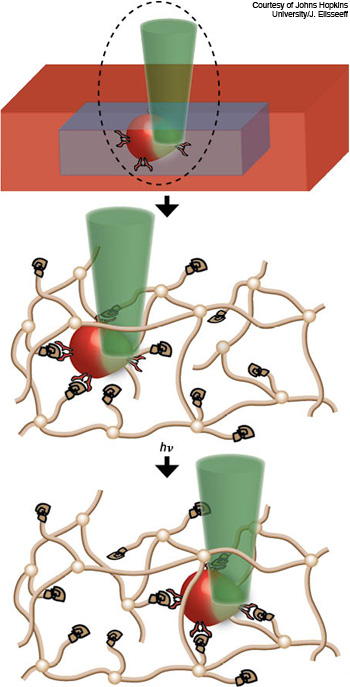 Biomaterial scaffolds: Reseachers realized spatio-temporal control of stem-cell migration in a PEG hydrogel network (brown lines) using laser pulses. A 458 nm laser (green) locally activated a protein to signal a pathway in the cells (red), triggering them to move in the direction of the light exposure through the biomaterial. A channel was created in real-time to provide space for the cells moving over a long distance.
Biomaterial scaffolds: Reseachers realized spatio-temporal control of stem-cell migration in a PEG hydrogel network (brown lines) using laser pulses. A 458 nm laser (green) locally activated a protein to signal a pathway in the cells (red), triggering them to move in the direction of the light exposure through the biomaterial. A channel was created in real-time to provide space for the cells moving over a long distance.