Detecting and treating cancer remains one of the biggest challenges in modern medicine. Physicians can significantly improve their patients’ chances of survival and quality of life with early detection and proper staging followed by a tailored treatment plan.
Up until now, biopsy has been the gold standard for making a definitive cancer diagnosis. Biopsy refers to the medical removal of a tissue sample from a living subject, followed by the analysis of thin slices (5-10 µm) of excised tissue under a microscope. Unfortunately, however, a physician can miss a tumor if it is still in an early stage (i.e., confined to a superficial layer of the tissue and thus not visible macroscopically).
As a result, biopsies can provide false negative results if they are not collected from the right locations. In addition, they almost never provide information about the degree of tumor penetration, which can be a decisive factor in helping doctors to choose the proper course of treatment.
Over the past few decades, new optical medical imaging techniques—including optical coherence tomography, nonlinear microscopy, confocal microscopy and others—have given physicians the information they need to do a biopsy in the right location. Yet they bring their own set of problems. For example, high-resolution imaging modalities typically possess a small field of view that can often only be extended through a time-consuming scanning process. Moreover, if image contrast enhancement agents are used, possible patient side effects must be considered.
 Abdelali Benali from the Institut Mutualiste Montsouris, France, and Angelo Pierangelo (sitting) from the École Polytechnique, France, take polarimetric images of an excised specimen of human colon with a Mueller polarimeter.
Abdelali Benali from the Institut Mutualiste Montsouris, France, and Angelo Pierangelo (sitting) from the École Polytechnique, France, take polarimetric images of an excised specimen of human colon with a Mueller polarimeter.
Fortunately, one of the intrinsic properties of light—its ability to be polarized—may one day provide a better solution. Our preliminary work indicates that ex vivo multispectral Mueller imaging may provide a useful way to quickly stage human colon cancer and to determine, within a few minutes, whether cancer treatment has been effective.
Polarized light and cancer
Besides the intensity values, polarized backscattered light can carry important information about a tumor’s properties. In general, the incident light is scattered and absorbed by biological tissue. The scattering process leads to the depolarization of reflected light. When one is looking for the abnormal tissue zones in dermatology, backscattered co-polarized and cross-polarized intensity images have better image contrast compared to unpolarized intensity images. But these orthogonal-state contrast linear or circular polarization images provide only part of the rich polarimetric information available.
In comparison, images that are the components of a complete Mueller matrix provide maps of the tumor sample’s depolarizing power, diattenuation and retardance for reflected light via suitable Mueller matrix decomposition. The resulting polarimetric image contrasts depend on the properties of the biological tissues. Usually, these contrasts will differ from those of unpolarized intensity images.
When a biological sample contains hemoglobin (a substance used by red blood cells to transport oxygen), its light absorption may change. This is another complicating factor that can affect the contrast of an image, because the extinction coefficient of hemoglobin varies significantly within the visible wavelength range. That is why the use of spectral information is of great importance for image contrast enhancement.
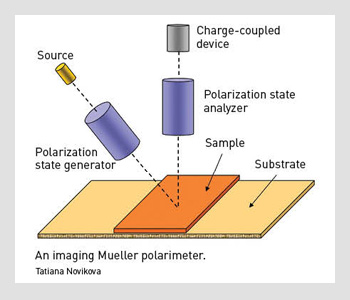 An imaging Mueller polarimeter.
An imaging Mueller polarimeter.
Mueller polarimetric imaging
With Mueller polarimetric imaging, at least 16 raw images have to be acquired with different input and output polarizations at each discrete wavelength. The instrument we designed to achieve this task has a wide field of view (FOV).
First, we illuminate the sample with a halogen lamp. The light beam passes through the polarization state generator (PSG), which consists of a polarizer and two nematic liquid crystals with fixed axes and variable retardations.
This setup allows efficient modulation of the incident beam polarization. The sample reflects that polarized light, which further passes through an analyzer that has the same optical elements as the PSG, but assembled in reverse order.
We used a telecentric illumination system to obtain a spatially uniform polarization state of the incident beam over the FOV. This choice eliminates artifacts caused by sample positioning uncertainty. We used a charge-coupled device camera with a resolution of 256 x 256 pixels to detect the backscattered light beam. A close-up lens coupled with the camera zoom made it possible to change the observation window from 4 to 25 cm². The wavelength was varied from 500 to 700 nm in steps of 50 nm using 20-nm-wide interference filters.
|
Optical staging
In our experiments, we opened human colon specimens along the axis of the colon tube and fixed them on a cork support. We took polarimetric images of the sample regions containing both healthy and cancerous zones. All images were captured with a multispectral imaging Mueller polarimeter. The typical ex vivo Mueller matrices of the human colon were essentially diagonal, indicating that colon tissue acts as a partial depolarizer. That is, it exhibits neither significant diattenuation nor retardance. As a result, only the M22, M33 and M44 elements of the Mueller matrix are relevant.
For both cancerous and healthy tissues, the relation |M22| = |M33| > |M44| holds true, meaning that the backscattered light is less depolarized when the incident light is linearly rather than circularly polarized. This is a typical signature of a Rayleigh regime in which the light is mainly scattered by particles that are small compared to the wavelength.
Our measurements were performed within the visible spectrum (500–700 nm), so the cell nuclei diameter (several µm) was much larger than the light wavelength. If the light is mainly scattered by cell nuclei, the incident circularly polarized light would be less depolarized when backscattered by the biological tissue. This is called a Mie regime.
As a result, the assumption that the cell nuclei are the major source of scattering by biological tissue in backscattering configuration does not hold true for the human colon. Our data provide evidence that light is mainly scattered by small objects (e.g., collagen fibrils, various subcomponents of cells). Cancer breaks the fine-ordered fabric of healthy tissue and initiates an increase in cellular density and vascularization within the diseased tissue.
The most relevant polarimetric images of ex vivo colon tissue are those quantifying the depolarization for incident linearly polarized light (M22 and M33 elements).
Our research demonstrated that cancerous areas that are both budding (earlier stage) and ulcerated (less thick and more advanced) depolarize less than healthy tissue at short wavelengths. However, the level of depolarization is not homogeneous within the ulcerated region. In the cancerous layer, histopathological examination revealed an increase in cellular density and vascularization with respect to the healthy zone, as well as modified morphological characteristics, the formation of stroma and the destruction of the natural order of the tissue.
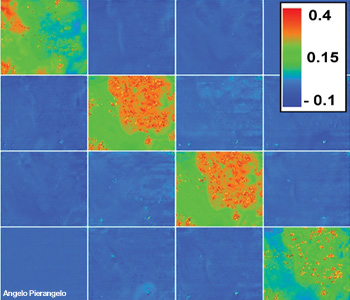 Typical Mueller matrix images of a cancerous polyp. The polyp is in the upper right corner of each square. The acquisition wavelength was 600 nm. The value of each pixel of polarimetric images was normalized by the value of corresponding pixel of the M11 image. The elements of both the third and fourth lines have an opposite sign.
Typical Mueller matrix images of a cancerous polyp. The polyp is in the upper right corner of each square. The acquisition wavelength was 600 nm. The value of each pixel of polarimetric images was normalized by the value of corresponding pixel of the M11 image. The elements of both the third and fourth lines have an opposite sign.
At all wavelengths the budding zone is always less depolarizing compared to all other parts. This means that the cancerous tissue with the high cellular density and vascularization typical of this region depolarizes less than the other tissues—a characteristic that is probably connected with the enhancement of both anisotropic scattering by large nuclei and light absorption due to the increase of tumor vascularization.
In the green part of the spectrum (at 500 and 550 nm), the ulcerated zone appears less depolarizing compared to healthy tissue, while this is no longer true at longer wavelengths. This is because of the increase of the light penetration depth with increasing wavelength, which is in turn due to the decrease of absorption by hemoglobin—a major source of light absorption in biological tissues within the visible wavelength range.
Because of strong hemoglobin absorption at shorter wavelengths, the polarimetric properties of backscattered light are defined by the most superficial tissue layers, which consist of cancerous tissue over all the budding and ulcerated zones, though with different thicknesses. At longer wavelengths, the signal may be dominated by scattering in the cancerous layer only where this layer is sufficiently thick. When the cancerous layer is thin, it is transparent for the red light, which is backscattered essentially within the muscularis and serosa layers, resulting in a polarimetric response similar to that of healthy tissue.
We also analyzed colon cancer samples from a patient treated with radio-chemotherapy (RC) before surgery. An endoscopic investigation before RC treatment revealed a tumor that had most likely penetrated the muscularis externa. After pre-operatory treatment and subsequent surgery, the polarimetric images of the excised specimen did not show any contrast between the ulcerated and healthy zones at all wavelengths, indicating that the RC treatment was successful. It was also confirmed by histopathological examination.
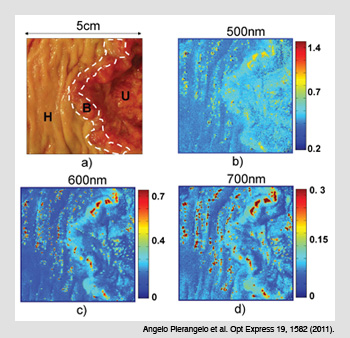 Polarimetric images of colon cancer. (a) The three zones of a common Liberkühn adenocarcinoma: (H) healthy tissue; (B) budding cancer; and (U) ulcerated tissue. (b) The spectral image of the M22 elements of the Mueller matrix at a shorter wavelength (500 nm) demonstrate that budding and ulcerated zones depolarize less than healthy tissue. (c-d) Images taken at 600 and 700 nm show that the depolarization is not homogenous in the ulcerated region.
Polarimetric images of colon cancer. (a) The three zones of a common Liberkühn adenocarcinoma: (H) healthy tissue; (B) budding cancer; and (U) ulcerated tissue. (b) The spectral image of the M22 elements of the Mueller matrix at a shorter wavelength (500 nm) demonstrate that budding and ulcerated zones depolarize less than healthy tissue. (c-d) Images taken at 600 and 700 nm show that the depolarization is not homogenous in the ulcerated region.
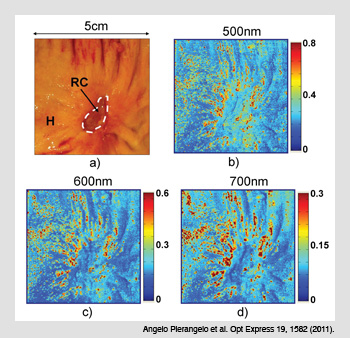 Cancerous colon treated with radio-chemotherapy. (a) Cancerous area treated with radio-chemotherapy (RC) and the surrounding healthy tissue (H). (b-d) The Mueller matrix images taken at three different wavelengths show no polarimetric differences between H and RC zones when the cancer is completely destroyed by RC.
Cancerous colon treated with radio-chemotherapy. (a) Cancerous area treated with radio-chemotherapy (RC) and the surrounding healthy tissue (H). (b-d) The Mueller matrix images taken at three different wavelengths show no polarimetric differences between H and RC zones when the cancer is completely destroyed by RC.
Looking ahead
We found that Mueller matrix polarimetric images of ex vivo human colon specimens with a specific form of colon cancer (Liberkühn adenocarcinoma) revealed enhanced contrast between healthy and cancerous sections. The measured depolarization depends on several factors, namely the presence or absence of a tumor, whether it is protruding outward or inward, its thickness (degree of ulceration), and its level of penetration into deeper layers of the colon. The specimen from a patient treated successfully with RC exhibited a uniform polarimetric response that is typical of healthy tissue in the area where the tumor was found initially.
Our work demonstrates that multispectral Mueller imaging can provide useful contrasts to quickly stage human colon cancer ex vivo and to look for the residual cancer after treatment. While this technique is still in a preliminary research phase, the results of our investigations are promising.
Spectroscopic and angular resolved Mueller matrix polarimetry has already proved its potential advantages for metrological applications in microelectronics and target detection in turbid media. Our work paves the way for the possible in vivo biomedical applications of the Mueller matrix imaging technique, particularly for:
-
the early detection of cancer.
-
improving the performance of biopsies.
-
cancer staging before surgery.
-
detecting residual tumors after radio-chemotherapy treatment.
-
detecting and monitoring tumor recurrence.
We still need to reduce the acquisition time by about 0.1 s, because unavoidable small movements (e.g., because of breathing) may affect the quality of the images. By conducting more experimental and theoretical studies of polarized light-tissue interaction, we will come to better understand the origin of the contrast in polarimetric images of biological tissue.
In some medical fields—including dermatology, gynecology and otolaryngology—the direct illumination of the imaging tissue is already possible. Otherwise optical fiber is needed to deliver the light to inner cavities within the human body. Thus, we must work to eliminate the polarimetric contribution of optical fiber to the acquired images. Research is progressing towards the development of an endoscope taking the macroscopic polarimetric images of the object with diffuse white light illumination, looking for high contrast regions and then probing the suspicious zones with laser beam (“optical biopsy”), as outlined in the opening illustration.
So, in summary, polarimetric Mueller matrix imaging maps a tumor sample’s depolarizing power, diattenuation and retardance for reflected light via suitable Mueller matrix decomposition. In doing so, this technique provides a wealth of important clinical information quickly and accurately; it holds promise to become a new optical tool—and perhaps one day a new standard—in clinical cancer care.
Tatiana Novikova, Angelo Pierangelo and Antonello De Martino, are with the Laboratoire de Physique des Interfaces et des Couches Minces, École Polytechnique, CNRS, Palaiseau, France. Abdelali Benali and Pierre Validire are with the département d’anatomopathologie de l’Institut Mutualiste Montsouris in Paris, France.
References and Resources
- The National Cancer Institute
- H.C. Van de Hulst. Light Scattering by Small Particles, Dover, N.Y. (1981).
- J.L. Pezzaniti and R.A. Chipman. Opt. Eng. 34, 1558 (1995).
- S. Huard. The Polarization of Light, Wiley, N.Y. (1997).
- G.W. Kattawar and M.J. Rakovic. Appl. Opt., 38(30), 6431 (1999).
- R. Kiesslich et al. Gastroenterology 127, 706 (2004).
- G. Zonios et al. Appl. Opt. 38, 6628 (1999).
- H.-J. Wei et al. World J. Gastroenterol. 11, 2413 (2005).
- T. Novikova et al. Appl. Opt., 45, 3688 (2006).
- S.L. Jacques et al. Proc. SPIE 6842, 68420I (2008).
- M.R. Antonelli et al. Biomed. Opt. Express 2, 1836 (2011).
- A. Pierangelo et al. Opt. Express 19, 1582 (2011).
- A. Pierangelo et al. J. Biomed. Opt. 17, 066009 (2012).

 Colon anatomy and cancer
Colon anatomy and cancer