
Natural and man-made coloring materials have been sought after and developed for thousands of years. In modern life, dyes are being used for an increasing number of commercial products, ranging from paper and fabrics to plastics and automobiles. Many well-established chemical companies started in business by manufacturing dyestuffs for a variety of industrial requirements.
Extensive research and development have gone into creating the very large number of dyes and pigments that are available on the market today. Traditionally, these products have been fabricated by making chemical modifications to certain classes of chemical compounds. For example, azo dyes were developed from nitrogen-containing aromatic compounds; they found widespread use during the 19th and early 20th centuries. The specific colors of such organic dyes are derived from the presence of color-conferring groups called chromophores.
Electronic transitions inside these groups cause them to selectively absorb radiation in certain ranges of the electromagnetic spectrum and thus appear colored to our eyes. Somewhat similar transitions within some inorganic compounds make them appear colored and thus useful as coloring pigments. All such materials that derive their specific colors from electronic transitions in atoms, ions and molecules can be referred to as chemical colorants.
In recent years, there has been a remarkable development in colorant technology. Weíve seen the advent of materials that derive their characteristic colors through the direct manipulation of light waves. These ìphysicalî colorants can be defined as color-conferring pigments that derive their spectral makeup from processes of physical optics, such as interference, diffraction, resonant light scattering, etc.
Nanotechnology plays a crucial role here, because the color-generating physics has its origin in the small-scale structures that these physical colorants possess. The technology of these materials is developing rapidly, with interest from both pigment manufacturers and end-users on the rise.
How physical colorants work
Physical-effect colorants derive their spectral makeup from the detailed way in which their structure is assembled at the nanometer scale. It is not difficult to see why specific nano-architectures could lead to their own chromatic effects. When ground to a fine powder, glass and other transparent materials appear white because of extensive inter-particle scattering, whereas very fine metal particles appear black because of uniform radiation absorption. Similar light scattering in ice grains cause calved icebergs to appear bluish in color.
These are examples of uncontrolled color generation. With careful nanostructural engineering, it is now possible to produce the desired surface colors. One advantage of engineering physical colorants is that they can be made to show color in any part of the infrared and visible spectral regions, and the color can be tuned through a simple scaling process. This is in spite of the fact that the pigment material itself may be completely colorless.
Often these structured materials can be easily customized and have characteristics and visual effects that are not possible to obtain from traditional pigments, such as pearlescent and metallic sheens, infrared-blocking properties and angle-dependent colors, which are useful in security applications. These physical colorants are well-suited for differentiating commercial products, and will be very attractive for a number of applications, such as automotive exterior coloring and novelty effects in cosmetics.
Color from thin-film interference
Thin-film interference has been used to produce some unusual color effects. This kind of color is readily seen from a water surface carrying an oil spill, as well as on coated camera lenses and oxidized silicon wafers. In order to produce a useful pigment, micron-sized chips of a suitable substrate material are coated with a series of inorganic thin films. Careful selection of coating materials, their thicknesses and layering schemes enable desired colors to be produced in transmission and reflection. Merck KGaA produces a family of interference-based pigments called Iriodin.
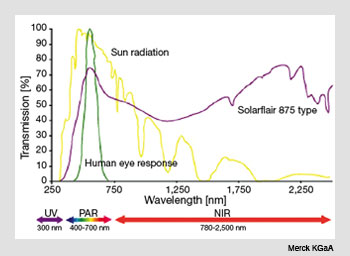 The transmission spectrum of Solarflair 875 pigment plotted with that of terrestrial solar spectrum and the response of the human eye. Notice the reduced transmission of infrared radiation in the near-infrared region.
The transmission spectrum of Solarflair 875 pigment plotted with that of terrestrial solar spectrum and the response of the human eye. Notice the reduced transmission of infrared radiation in the near-infrared region.
This material is a multilayer synthetic pigment consisting of basal mica coated with silicon dioxide (SiO2), titanium dioxide (TiO2) and tin oxide (SnO2) overlayers. Weather-resistant grades are also available that contain an additional coating of zirconium dioxide (ZrO2). This pigment is temperature stable up to 800° C.
An extended version sold under the brand name Solarflair is also available. This material has been especially engineered for reflecting infrared radiation. The two main commercial offerings for interior applications are called Solarflair 870 and Solarflair 875. These pigments have particle sizes in the range of 10-60 mm and 5-25 µm, respectively.
The former is suitable for general purpose infrared-blocking applications, while the latter is tailored to have a transmission spectrum that matches photosynthesis requirements of vegetation quite closely and is thus suitable for use in green houses.
The figure on the right shows the transmission spectrum of Solarflair 875 together with that of the solar spectrum at the Earthís surface. The actual pigments are inert, non-toxic, off-white powders, but these can be blended in plastics and glasses in various concentrations to produce the desired infrared-blocking effects.
 Xirallic F60-50 SW Fireside Copper interference-based pigment.
Xirallic F60-50 SW Fireside Copper interference-based pigment.

Gleam and glitter from new effects pigments.
Merck developed this family of pigments for a number of applications, ranging from the production of pearlescent and metallic sheen effects, which are used in pigments that are sold to paint manufacturers, to the reflection of heat-producing infrared radiation from enclosed spaces. Other related product families from Merck include Colorstream, Xirallic and Miravalä. The figure on the rightt shows a specimen of Xirallic Fireside-copper pigment.
Ciba speciality chemicals offers a similar product line called Xymara effect pigments. Again, these are composed of multi-layered coatings of high-refractive-index materials, such as titanium and iron oxides on thin mica platelets with thickness in the range of 100 to 500 nm. Different particle sizes cause different visual effects: 5- to 25-µm-wide platelets give a silky gloss effect; 10- to 50-µm platelets yield a brilliant effect; and 30- to 150-µm platelets are used for providing glitter effects. The Xymara family of pigments have become popular for many uses, including cosmetics and car body paints.
All these "effect" pigments could be used either as standalone pigments or in combination with other organic or inorganic dyes. Used with other coloring materials, they enhance the visual appeal of a painted product by imparting the desired glossy or glittery sheen to the colored surface. Example applications are illustrated in the figures on the right, which show their use with plastics and for producing metallic-looking plastic wires for decorative applications.
 Xymara gold color effect on plastic wires.
Xymara gold color effect on plastic wires.
Diffractive effects
Diffraction can also be used for producing striking color effects. The play of colors reflecting off a diffraction grating is well known. When diffractive structures are engineered at a small enough scale, multicolor pigments can be produced. Thin metal flakes form a suitable substrate for producing such pigments. Metal flake-based metallic effect pigments are available from companies such as Ciba Inc., whose Metasheen family of pigments is widely used in the print and packaging industry.
These vacuum-metallized pigments are composed of lamellar, non-leafing aluminum flakes dispersed in a variety of solvents. The modification of metal flakes by corrugating them produces interesting diffractive color-spill effects. The structure of a micro-embossed diffractive metal film can be seen in the figure on the facing page, which is a scanning electron micrograph of a corrugation-patterned aluminum film. Pigments derived from structured metal flakes can have applications in security-enabled and anti-counterfeiting products such as bank notes, passports and credit cards. The particular arrangements of metal flakes scatter light in a characteristic pattern and thus act as fingerprints for authentic documents; this ensures that the authenticity of the documents can always be verified.
Nanoparticles using surface plasmons
When light interacts with metal particles, it excites surface-free electrons to produce collective electron density oscillations called localized surface plasmons. These resonant excitations are extremely sensitive to the shape, size and dielectric environment of the nanoparticles. Consequently, light scattered from specifically shaped metallic nanoparticles embedded in suitable dielectric hosts can give rise to anisotropic color effects.
In contrast to other types of physical-effect colorants, nanoparticles that use resonant plasmon effects can exhibit different spectral signatures depending on whether they are illuminated by polarized or unpolarized radiation. An ancient example of the use of plasmons is in stained glass. Plasmons on metal particles such as gold can absorb blue and yellow light but reflect red to give a characteristic ruby color. Similar effects are possible with copper for blue and green and iron for green and brown.
Compared to other physical pigments, this class of materials is harder to prepare but offers far more control over the characteristics of scattered radiation. Plasmonic pigments have only been investigated in the laboratory so far and appear to be several years away from commercial development. However, due to their extreme customizability, these materials promise an unusually large range of practical applications. Itís little wonder, then, that surface resonant plasmon particles are a topic of great scientific interest these days.
Photonic-crystal-based color
Yet another nanostructured system capable of producing a wide range of color effects is one made from artificial periodic structures called photonic crystals. Essentially, these are made from an ordered alternating arrangement of at least two constituents with different refractive indices. They can be in the form of patterned ridges or pillars, spheres or the inverse of these structures. The usual defining feature of a photonic crystal is the regularity or periodicity of the structure in terms of its refractive index.
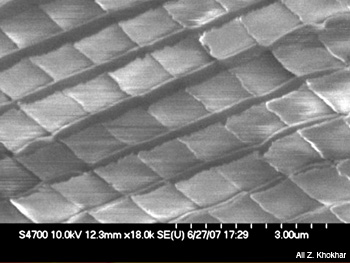
Scanning electron micrograph of micro-embossed diffractive aluminum film.
For instance, a close-packed arrangement of polystyrene spheres and the air between them form a three-dimensional periodic array of high (polystyrene) and low (air) refractive index material. This arrangement leads to the emergence of wavelength or frequency ranges where light cannot propagate—a so-called photonic bandgap. This is similar to the formation of allowed bands and band gaps for electron waves in semiconductor crystals.
The major difference is that, in photonic crystals, the constituents are much larger than single atoms and, correspondingly, these materials work with longer wavelengths of electromagnetic radiation. Depending on the arrangement and refractive-index contrast between the nano-constituents, light propagation is stopped in one direction or all directions. In the visible range, a partial stop band manifests itself as a strong reflection at a particular wavelength, which may change with the angle of tilt—a so-called play of colors.
This effect can be observed in opal—a natural three-dimensional photonic crystal—which consists of submicron-sized silica spheres. Other natural examples include some butterfly wings and sea creatures, which have nano-structured features in their wings and scales. Indeed, the richness of iridescent colors seen on butterfly wings hints at the effects that are possible with photonic-crystal-based surface coatings.
At the present stage of technological development, photonic crystals can be coated on large glass and plastic substrates to produce attractive glazing products with obvious architectural applications. This is usually done through a self-assembly approach, in which a slurry of size-selected silica or polystyrene balls is allowed to dry on a substrate in a controlled atmosphere. Further work is in progress to develop commercial oil and water-based dispersions of photonic crystallites for use in surface coating applications.
In an exciting recent development, researchers have demonstrated that they can change the color of photonic crystals by altering the spacing between the nano-constituents—for example, by applying pressure. This can be achieved permanently or reversibly for small shifts of less than the elastic limit of the films, allowing selective area "stamping" of color onto the photonic crystals. It also opens up an entirely new area of pressure-sensitive colorants that can find applications in the security and transportation industries.
In addition, the structural effects of photonic crystals can be used in combination with conventional dyes and inorganic nano-dot emitters, such as cadmium telluride (CdTe), which can be incorporated into the interstices of chosen photonic crystals. This generally allows shifting and modification of the emission by the photonic bandgap.
Two-dimensional photonic crystals are easier to fabricate using lithographic processes and nanoimprint methods and can be used to control the emission of light from LEDs and nano-dot structures, with the aim of increasing their efficiency. Here, the stopband is not the important parameter in extracting the light. Rather, what matters is the detailed interaction with the band structure; a flat band near the gamma point allows light to couple perpendicularly out of the structure when the photonic crystal is correctly scaled.
Placing photonic crystals directly on top of light emitters is now a rapidly maturing area of optical source design and is already resulting in commercial flat panel displays with brighter and richer colors. Thus, photonic crystal structures are useful for color-control purposes with both far-field and near-field light coupling.
We are witnessing the emergence of new classes of coloring materials that are very different from the dyes and pigments of yesteryear. These pigments will lead to future products that have striking visual effects and enhanced functionalities. By all indications, we are just starting to investigate the possibilities inherent in manipulating light and color through the use of nanostructured materials.
This is one area of nanophotonics that is under close scrutiny by both industrial and academic research labs. At this point, many possibilities are being investigated, with some already making their appearance as successful commercial products. As more innovative products are developed, the rainbow of applications for physical-effect pigments will expand even further.
[ Faiz Rahman and Nigel P. Johnson are with the department of electronics and electrical engineering at the University of Glasgow, Scotland, United Kingdom. ]
References and Resources
>> C.F. Bohren and D.R. Huffman. Absorption and Scattering of Light by Small Particles, Wiley, N.Y., 1998.
>> G. Pfaff and P. Reynders. "Angle-dependent optical effects deriving from submicron structures of films and pigments," Chem. Rev. 99, 1963 (1999).
>> M.S. Reisch. "Rainbow in a can," Chemical and Engineering News 81, 25 (2003).
>> J. Henzie et al. "Manipulating the optical properties of pyramidal nanoparticle arrays," J. Phys. Chem. B 110, 14028 (2006).
>> A.Z. Khokhar et al. "Imprinting on synthetic opal by applying uniaxial pressure," Micro. Nano. Lett. 1, 43 (2006).
>> C.L. Nehl et al. "Optical properties of star-shaped gold nanoparticles," Nano. Lett. 6, 683 (2006).
>> F. Rahman and R.M. De La Rue. "Photonic crystals enable ultra-high-brightness LEDs," Photonics Spectra, p. 52, July 2007.
>> G. Pfaff. Special Effect Pigments, William Andrew, ISBN: 0815515669, 2007.
>> A.Z. Khokhar et al. "Modified emission of semiconductor nano-dots in three-dimensional photonic crystals," IET Circuits Devices and Systems 1, 210 (2007).
>> A.Z. Khokhar et al. "Permanent tuning of the opal stop-band with the application of uniaxial pressure," J. Opt. A.: Pure Appl. Opt. 9, 446 (2007).
