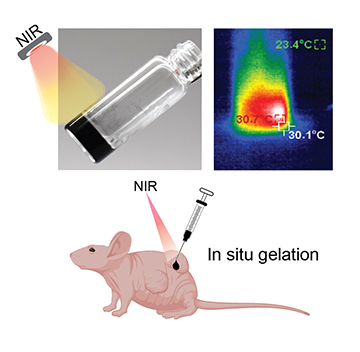
A visual representation of how light-responsive hydrogels absorb and convert near-infrared light to heat, which can be developed to control thermoresponsive materials. The precursor solutions form hydrogels upon NIR irradiation after being injected into the tissue beneath the skin of mice. [Image: A. Gaharwar/Texas A&M Engineering] [Enlarge image]
Since the invention of the first synthetic hydrogels in 1954, these water-swollen polymeric networks have found applications across industries as diverse as agriculture, pharmaceuticals and cosmetics. Hydrogels are produced by the simple reaction of one or more monomers, and their structure exhibits the ability to retain water without dissolving.
The higher biocompatibility of hydrogels relative to other materials has made them attractive for biomedical uses like biosensors, drug-delivery vectors and matrices for tissue engineering. Now, researchers at Texas A&M University, USA, have created a light-responsive hydrogel triggered by near-infrared light, which has the advantage of penetrating deeper into biological tissue than other wavelengths (Adv. Mater., doi: 10.1002/adma.202101238).
Advancing photomedicine
Most light-responsive hydrogels are activated by ultraviolet or visible light, meaning that penetration depth remains limited to less than 1 mm. For example, photothermal and photodynamic therapies employ light and a photosensitizer to induce cell death for the treatment of cancer and other medical conditions. However, the organic photosensitizers approved by the U.S. Food and Drug Administration are triggered by short-wavelength light that can only reach superficial tissue.
Akhilesh Gaharwar and his colleagues wanted to develop an inorganic nanomaterial compatible with near-infrared light, which penetrates further into biological tissue, for the next generation of photomedicine.
“Light-responsive inorganic biomaterials are an emerging class of material that can be used for non-invasive, non-contact and controllable medical devices,” said Gaharwar, an associate professor of biomedical engineering at Texas A&M. “And near-infrared radiation can penetrate deep inside the tissue and doesn’t have any side effects. So we wanted to develop something based on these two technologies.”
On-demand drug delivery
The researchers started by synthesizing molybdenum disulfide—a 2D nanomaterial with strong near-infrared absorbance—with atomic defects. When made to interact with a temperature-responsive polymer network, the atomic defects created more active binding centers to form the final crosslinked, self-assembled hydrogel. Activated with near-infrared light, the molybdenum disulfide nanoparticles heat up rapidly, and the temperature change triggers irreversible gelation of the hydrogel.
To further test the biomaterial, Gaharwar and colleagues made a hydrogel sample encapsulated with doxorubicin, a common chemotherapy drug. Illumination with near-infrared light successfully triggered gelation and shrinkage in the hydrogel underneath the skin of a mouse, leading to the on-demand release of doxorubicin. The release profile can be controlled by varying the intensity of light and time of exposure.
Exploring clinical applications
According to the team, the results represent the first time that near-infrared light has been used to activate gelation of self-assembled hydrogel without the addition of chemical reagents. Next, Gaharwar looks forward to exploring the clinical applications of the technology with more collaborative biomedical research.
“We are working with some folks at the Houston Medical Center to develop this particular material for cancer therapeutics,” said Gaharwar. “It is still in early stages, and we are testing out the technology in animal models first.”