![]()
A team of researchers in Singapore and China has developed a microlaser whose emission wavelength can be shifted by introducing single-stranded DNA into the cavity. The switching works by reorienting liquid crystals that form part of the laser gain medium. [Image: Courtesy of Yu-Cheng Chen]
As lasers have gotten smaller, entering the microscale and nanoscale, the creation of similarly fine-grained mechanisms for controlling and switching them raises some interesting engineering problems. Now a team of researchers at Nanyang Technological University (NTU), Singapore, and Taiyuan University of Technology, China, has created a microlaser whose emission wavelength can be simply and rapidly switched by adding a little DNA (ACS Nano, doi: 10.1021/acsnano.0c08219).
The team’s “DNA self-switchable microlaser” involves a micro Fabry-Pérot cavity, with a dye-doped liquid-crystal matrix within the cavity acting as the gain medium. Because of some clever surface chemistry in the matrix materials, the introduction of single-stranded DNA (ssDNA) into the cavity changes the orientation of the liquid crystals, and thereby shifts the laser’s emission wavelength. What’s more, introducing another strand of DNA complementary to the first ssDNA sequence reverses its effect, switching the laser back to its original wavelength.
The researchers believe that the work offers possibilities for the control of tiny coherent light sources that leverage the complexity and specificity of biomolecules. The team even sees potential applications in niche biosensing areas and in data encoding and storage—though these applications look to be some distance off.
DNA as control knob
The leader of the research team, Yu-Cheng Chen at NTU, notes that optical switching generally involves fabricating complex additional devices or finding ways to change the structure or refractive index of the lasing cavities themselves. For microlasers, DNA—the information-rich molecule that drives genetics—seems a natural candidate for a control knob, given its size and intrinsic programmability.
Indeed, various research groups have examined the use of DNA interfaces for controlling light–matter interactions—including the use of fluorescently labeled DNA and specially structured “DNA origami” to control specific light-emitting sites. These approaches, though, require advance work labeling the DNA, or designing specific, potentially fragile DNA structures in the case of the DNA origami approach.
The switchable-microlaser concept that Chen’s team has developed instead rests on the use of robust, natural DNA—specifically single-stranded DNA. As the name implies, ssDNA represents only one half of the celebrated DNA double helix. When an individual strand of ssDNA meets up with a strand of complementary DNA (cDNA)—another piece of ssDNA with a sequence of base pairs precisely complementary to the first—the pair will join or “hybridize” into double-stranded DNA in the classic helical pattern.
Creating the microlaser
To build a microlaser that could use DNA hybridization as a switching mechanism, Chen’s team began by creating a gain medium that could be chemically switched by interaction with ssDNA. The researchers mixed commercially available nematic liquid crystals, 0.3-weight-percent laser dye, and a long-chain surfactant, octadecyltrimethylammonium bromide (OTAB), to create a liquid-crystal solution. The OTAB is the “secret sauce” in the experiment; it forms a monolayer coating around the liquid crystals that can bind to polyanionic molecules such as ssDNA, allowing potential control of the crystals’ orientation.
Next, the team fashioned two tiny dielectric mirrors to create a 150-µm Fabry-Pérot cavity, and introduced 1.5 microliters of the liquid-crystal solution into the space between the mirrors. The researchers pumped the cavity with 470-nm light from a pulsed laser, and confirmed that the cavity lased, with an emission peak in the area of 619 nm.
Blue-shifting and back again
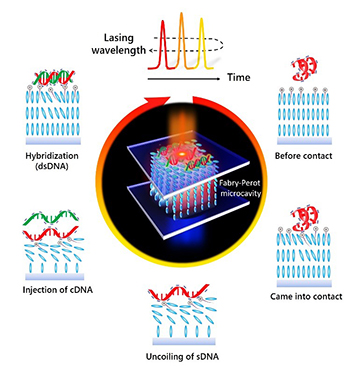
Under the switching scheme, ssDNA binds to the liquid crystals, changing their orientation and the cavity’s optical properties and blue-shifting the lasing wavelength. The addition of complementary ssDNA, or cDNA, causes the two single strands to combine into double-stranded DNA, breaking the bond with the liquid crystals and restoring the laser to its original wavelength. [Image: Courtesy of Yu-Cheng Chen]
When ssDNA is added to the matrix, it uncoils and binds to the thin veneer of OTAB around the liquid crystals, causing the liquid crystal molecules to flatten. The change in the liquid-crystal orientation slightly alters the absorption cross-section of the dye molecules in the gain solution, leading to a small but measurable blue-shift of the lasing wavelength, to around 615 nm.
Next, when ssDNA with the complementary sequence—that is, cDNA—is injected into the cavity, the two complementary single strands hybridize into twisted, double-stranded DNA. That breaks the chemical binding to the liquid crystals, allowing them to reorient and red-shifting the lasing wavelength back to its original value. The team found, moreover, that the rate at which the switching took place depended on the concentration of the DNA molecules used.
From biosensors to information coding?
In an email to OPN, Chen said he believes that the work offers “key insights for bioinspired photonic devices that take advantage of the self-recognition or self-regulation of biomolecules and cells to modulate light at the sub-nanoscale.” Looking out somewhat further, Chen envisions some potential applications. In biosensing, for example, he suggests that the same concept, using targeted RNA sequences rather than ssDNA ones, could conceivably be used to identify the presence of RNA from certain viruses through observation of the laser switching.
Perhaps more speculatively, Chen also sees potential uses of DNA’s capabilities as a laser switch in schemes for encoding and storing information. He admits that “this may seem still far from reality in nowadays,” but believes that “the concept is truly possible and possess the great potential in optical encoding through micro-nano lasers.”
