![]()
Illustration of a rat with the head-mounted optical cochlear implant system. [Image: Institute for Auditory Neuroscience, University Medical Center Göttingen]
More than 5% of the global population lives with disabling hearing loss. In some cases, this can be treated with a cochlear implant—an electrical neuroprosthesis inserted inside the ear to stimulate the auditory nerve. This device can restore hearing to some extent but provides users with limited frequency, or spectral, information.
Researchers in Germany have combined gene therapy with an optical cochlear implant (oCI) of their own design to improve the quality of bionic hearing with cochlear implants (Sci. Transl. Med., doi: 10.1126/scitranslmed.abb8086). In their proof-of-concept study, the researchers demonstrated hearing restoration in rodents using their LED-based multichannel optical system—and they have their sights set on a 2025 human clinical trial.
Can you hear me now?
Deafness is a widespread impairment caused by dysfunctional or missing sensory hair cells in the cochlea—the snail-shaped cavity of the inner ear. In a normal functioning auditory system, sound in the form of vibrations will enter the ear and move the hair cells, which then convert the vibrations into nerve impulses that are sent to the brain and interpreted. Electrical cochlear implants (eCIs), which are used by about 700,000 people worldwide, bypass the sensory hair cells in the ear and directly stimulate the auditory nerve electrically—using 12 to 24 electrode contacts covering a frequency range of about 100 Hz to 10 kHz.
Think of the cochlea as a spiral staircase where every step corresponds to a frequency, says Tobias Moser at the University of Göttingen, Germany, a coauthor of the recent study. The neurons responsible for coding high frequencies are situated in the base of the cochlea, and those responsible for low frequencies reside in the top.
The goal, Moser says, is to stimulate very few neurons or steps at a time to maximize frequency resolution. But the saline in the cochlea conducts electricity, and the broad spread of current from the electrode contacts of eCIs tends to activate many neurons of the ear simultaneously, causing channel crosstalk and affecting the quality of a person’s restored hearing.
“This is considered the major bottleneck of the eCI,” says Moser, “and despite efforts such as multipolar stimulation and current steering it seems hard or even impossible to overcome.”
Light versus electricity
A silicone-molded optical cochlear implant undergoing a bending test. [Image: D. Keppeler et al., Science Translational Medicine (2020)]
Moser’s idea to address this unmet clinical need was inspired by the advent of optogenetics in the early 2000s. If the neurons in the ear could be modified to respond to light rather than just sound vibrations, he recalls thinking, “then perhaps photonic stimulation could transform bionic hearing, since light can be better confined in space than electric current.”
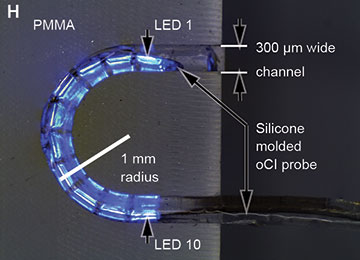
First, Moser and colleagues used a harmless virus to genetically modify neurons in the ears of rats and gerbils to express the light-activated ion channel known as channelrhodopsin (ChR). Then, the team partnered with researchers at the University of Freiburg, Germany, to design a µLED-based oCI. The multichannel device contains 10 power-efficient blue LED chips, with an LED pitch of either 350 µm or 500 µm, integrated on a polyimide carrier. The researchers engineered the oCI to be slim and flexible—no more than 300 µm wide and with a minimal bending radius of 1 mm.
Before implanting the devices in rodents, the team studied the electrical, optical and thermal properties of the implant in vitro, and optimized it for rodent ears. Next, the researchers inserted the device into transgenic rats and characterized the oCI’s stimulation of the animals’ auditory nerves. To characterize the frequency selectivity of the oCI devices, says Moser, “we compared the spatial spread of excitation from the LEDs of the oCI to that from electrodes of an eCI in the same ear/animal by recordings from the midbrain.”
In behavioral experiments, the team began with rats with normal hearing and trained them to perform a behavior driven by sound. After deafening the rats, the team implanted the oCI device, finding that the animals could still perform the acoustically trained behavior with the aid of the multichannel implant. According to Moser, “this is the first demonstration and characterization of preclinical hearing restoration by a complete multichannel oCI system based on LEDs.”
In the clinic
Used in conjunction, the gene therapy and optical implant reportedly demonstrated an improved frequency selectivity compared with the electrical alternative. Encouraged by these results, the team has spun-off a company, OptoGenTech, to work toward getting the gene therapy and oCI system into a human clinical trial and, eventually, onto the market.
Moser notes that while the study provides a first proof of concept of multichannel optogenetic stimulation, the approach and oCI design must be optimized before proceeding to clinical trials. Establishing and characterizing arrays with a higher density of smaller and highly efficient thin-film mLEDs will be an important next step. The team also intends to increase the viral transduction rate to express ChR in most, if not all, neurons of the ear, as well as devise a tailored optical-stimulation strategy for sound coding.
