
In a laboratory experiment to optimize electricity production, EPFL scientists reproduced the real-world conditions of pH and salt concentration that occur where rivers meet the sea. [Image: iStock]
Estuaries—where freshwater rivers meet and mingle with the briny oceans—carry the potential for producing continuous, renewable energy yet to be tapped by humans. Researchers at a Swiss university have developed a light-optimized osmotic process that could harness the energy of naturally occurring estuaries (Joule, doi: 10.1016/j.joule.2019.04.011).
Specifically, the team at the École Polytechnique Fédérale de Lausanne (EPFL) discovered that light excites electrons in an extremely thin semiconductor ion-exchange membrane. This effect roughly doubles the amount of electrical energy that can be extracted from the setup.
Blue energy
Beachgoers and boaters enjoying a large estuary such as the Chesapeake Bay in the United States or the Haliç (Golden Horn) in Turkey may not realize that they’re floating on a potential source of osmotic power, sometimes dubbed “blue energy.” Fresh and salt waters have a chemical potential difference that in principle stores enormous quantities of energy. Two processes exist to extract that energy as electricity—reversed electrodialysis and pressure-retarded osmosis—but they are not yet useful for regular electricity-generating facilities.
Three years ago, Aleksandra Radenovic and her team at EPFL’s Laboratory of Nanoscale Biology developed a molybdenum disulfide membrane that could convert salinity gradients into electricity. Those experiments, however, were conducted under highly artificial conditions. Back then, the group used strongly alkaline solutions to boost the surface charge on the membrane and thus enhance its efficiency, says Michael Graf, an EPFL doctoral candidate and lead author of the report. Now the team has demonstrated that light can have the same effect on the membrane while it is immersed in more neutral-pH liquids (the pH of typical estuary waters is around 7.4).
Nanopores and atomically thin membranes
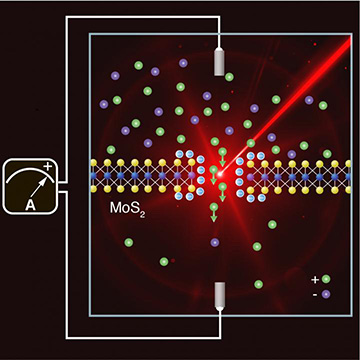
Reproducing the conditions that occur at estuaries, EPFL scientists shined light on a system combining water, salt and a membrane just three atoms thick to generate more electricity. [Image: EPFL]
The researchers fabricated an MoS2 membrane only three atoms thick and drilled a “nanopore” a few nanometers wide into the middle. While it was immersed in solution with different salinities on either side, the team irradiated the membrane and its tiny hole with light from two diode lasers, one with a wavelength of 643 nm and the other with a wavelength of 475 nm. The group kept the irradiation below 1.5 W/cm2 to avoid poking additional holes in the membrane, and found that 10 nm was a better pore size for current generation than 3 nm.
The team’s biggest challenge, according to Graf, was maintaining the long-term stability of the three-atom-thick MoS2 membrane, which depends on the mechanical properties of its substrate. Graf says that he and his colleagues carefully designed the substrate to expose only a fraction of a square micron at a time to the light and water.
Graf estimates that the semiconductor membrane process could yield thousands of watts per square meter of membrane—as opposed to roughly 200 W/m2 from traditional solar cells. The size of the estuary doesn’t matter, he adds, because river and sea water would be diverted into small mixing reservoirs separated by the membrane at the osmotic-power station.
Scaling the solution
For any real-world estuary, the group must produce a membrane that is larger than a single nanopore, according to Graf. He says he’s confident that rapid progress in 2-D materials science will lead to larger sheets of MoS2 membranes or other types of semiconductor or plasmonic membranes.
Under the effect of light, the system produces twice as much power as it does in the dark. [Image: EPFL]
“We currently don’t understand how the power output scales with the number of pores introduced into the membrane,” Graf adds. “Therefore, the next steps are to investigate the behavior of porous membranes to find out how the energy production scales with different densities of nanopores. Furthermore, we need to develop further ways of stabilizing the membrane so that it can be used over a long period of time in a natural environment.”
Earth receives about 100 mW/cm2 of solar energy at sea level during the daytime, so the EPFL group envisions that the type of concentrators now used in solar cells could direct sunlight to the membrane-based generators in future estuary “blue energy” plants.
Researchers from the University of Illinois at Urbana-Champaign, USA, also contributed to this study.
