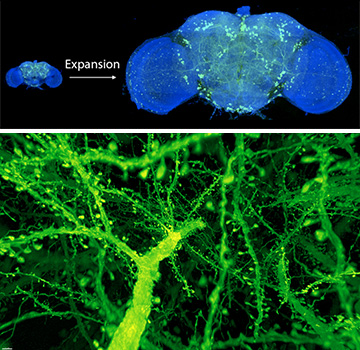
Top: After expanding the fruit fly brain to four times its usual size, scientists used lattice light-sheet microscopy to image all of the dopaminergic neurons (green). Bottom: A forest of dendritic spines protrudes from the branches of neurons in the mouse cortex. [Images: Gao et al., Science 2019]
Sometimes, a little stretch yields enormous results. By chemically expanding brain tissues and subjecting them to advanced microscopy techniques, U.S. scientists have produced three-dimensional images of neuronal circuits with nanoscale resolution (Science, doi:10.1126/science.aau8302).
The research team resolved the entire brain of a fruit fly (Drosophilia) and a cortex-wide chunk of a mouse’s brain down to a resolution of 60 nm by 60 nm by 90 nm. At that level of detail, scientists can calculate the volume of organelles, measure variations in myelination among neurons and trace the dendritic spines that protrude from these neurons.
Stretching and illuminating
Electron microscopy has provided some of these tiny details from small sections of brain tissue, but the technique cannot distinguish specific proteins. Plus, the method is slow. Other modern microscopy techniques have additional drawbacks, such as cellular phototoxicity, that would interfere with the team's goal: nanoscale mapping of large swaths of animal brains.
First, the scientists prepared the brain specimens with a chemical-expansion technique developed by, among others, neuroscientist Edward S. Boyden of the Massachusetts Institute of Technology (MIT), USA. Called expansion microscopy, the method involves infusing the tissue with a polyacrylamide/polyacrylate gel containing fluorophores, then expanding the gel in a water solution. Boyden and his colleagues blew up the brain tissue by a factor of four in each dimension; expanding it further would have required higher densities of fluorescent labels.
After this process, the researchers were dealing with a volume 64 times larger than the original fly brain, which would have taken an enormous length of time to visualize at the molecular level. To hasten the process, they employed lattice light-sheet microscopy, a technique developed by team member and Nobel laureate (and OSA Life Member) Eric Betzig, University of California Berkeley, USA, and Howard Hughes Medical Institute (HHMI), USA. Lattice light-sheet microscopy involves sending a sheet of light—a stretched, linearly polarized laser beam—through a labeled specimen and recording the fluorescence from the specimen on a high-speed camera.
Sweeping these light sheets through a brain sample reduces photobleaching of the fluorescent labels—but acquiring images at roughly 100 million voxels per second creates huge data sets. The team had to create a terabyte-scale data-processing pipeline for applying flat-field corrections, stitching together images and visualizing the resulting 3-D data.
Fast forward
Imaging the fly brain in multiple colors took the research group 62.5 hours (2.6 days)—but it would have likely taken years with a standard electron microscope. The scientists hope that the accelerated imaging capability will enable future comparative studies of multiple brains to reveal gender differences, neural development stages and other topics of interest.
Besides Boyden (also an HHMI researcher) and Betzig, the team included MIT postdoctoral fellows Ruixuan Gao and Shoh Asano, Srigokul Upadhyayula of Harvard Medical School, USA, and representatives of four other U.S. laboratories.
