
Irradiation setup to switch a photoswitchable signaling molecule from the trans-isomer to the active cis-isomer. [Image: Dusan Kolarski / University of Groningen]
A research team from the University of Groningen, the Netherlands, reports synthesizing and testing a series of 16 photoswitchable signaling molecules that can be used to externally control quorum sensing (QS)—a cell-to-cell communication method bacteria use to react to their environment and to regulate gene expression (Chem, doi: 10.1016/j.chempr.2019.03.005).
External control of QS with light could, say the researchers, lead to a better understanding of QS mechanisms and potentially new clinical approaches to treating serious bacterial infections, particularly those that are resistant to antibiotics.
Stable, reversible light switch
During QS, bacteria produce, excrete and detect signaling molecules called autoinducers. These autoinducers can communicate a number of things to the surrounding bacteria (for example, something dangerous in their environment) and trigger a response in the form of gene expression (for example, releasing a toxin or forming a biofilm). QS is an interesting target for photopharmacology because it could lead to the ability to control bacteria’s biological functions with easy-to-manipulate and usually non-toxic light.
While researchers have already succeeded in attaching molecular “light switches” to QS autoinducers, these methods are typically low-yield and result in unstable molecules. Now, the Netherlands team, led by Ben Feringa, has developed a simpler approach to creating these light-sensitive molecules. The team’s two-step high-yield method relies on a coupling reaction with a stabilized intermediate to produce stable, photoresponsive QS autoinducers.
Using this two-step synthesizing method, Feringa and his colleagues created a library of 16 different photoresponsive autoinducers that could be used to inhibit or stimulate QS in Pseudomonas aeruginosa (a bacterium that causes pneumonia). Specifically, the photocontrolled autoinducers are engineered variations of N-acyl homoserine lactone (AHL) molecules with a light-sensitive azobenzene motif that acts as the light switch and is located between the head of the molecule and its four-carbon tail.
Testing photopharmacological behavior
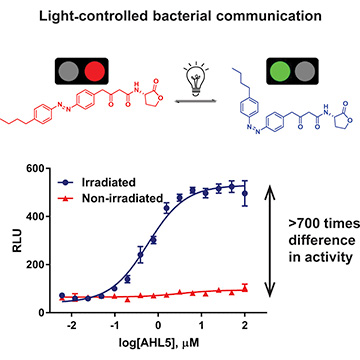
The photoswitchable molecule AHL5 changes shape when irradiated with light, from its trans-isomer inhibitor form (top left) to its cis-isomer activator form (top right). There is a 700-fold difference in activity between the AHL5 trans- and cis-isomers (bottom). [Image: Reprinted from Chem, doi: 10.1016/j.chempr.2019.03.005; Copyright (2019), with permission from Elsevier.]
The researchers characterized the photochemical properties of each of the 16 photoresponsive autoinducers (named AHL1–AHL16). They found that when the photoswitches are irradiated with light at 365 nm, the “tails” bend, forming the cis-isomer forms of the molecules and stimulating a QS signal. When the light source is removed, the photoswitches reverse back to their trans-isomer (straight tail) forms. And, depending on the autoinducer, the trans-isomer form either has no effect on the QS signal or it inhibits QS.
One of the autoinducers, AHL5, was particularly interesting to the team. During testing, they found that the AHL5 autoinducer could both stimulate and inhibit a QS response, and it exhibited a 700-fold difference in activity between its cis- and trans-isomer forms. The researchers say that this degree of reversibility and change in activity has, to their knowledge, never been seen before in a light-switch molecule. They also observed that cis- and trans-isomer forms of AHL5 could control toxin production in live P. aeruginosa.
Based on the results from the characterization tests and demonstrations with P. aeruginosa, the University of Groningen team believes that AHL5 and the other photoresponsive autoinducers are “highly promising as next-generation light-controlled research tools” for investigating QS mechanisms and clinical photopharmacology applications.
