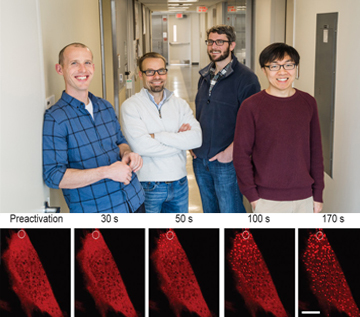
Princeton University researchers create an optogenetic tool to view protein condensation in vivo; pictured left to right are team members Jared Toettcher, Mikko Haataja, Clifford Brangwynne and Yongdae Shin (top). Time-lapse images of protein-cluster wave formation appear as light dots as a cell is exposed to a blue laser (bottom). [Image: Princeton University] [Enlarge image]
Membraneless organelles play an important role in the processing of genetic information in the cell. However, scientists don’t understand how the proteins that form these organelles assemble and stick together in the surrounding intracellular soup. Now, a group of U.S. researchers from Princeton University has used optogenetically modified intracellular proteins to study in vivo phase-changes that mimic the physiology of membraneless organelles (Cell, doi: 10.1016/j.cell.2016.11.054).
Corresponding author Clifford Brangwynne says the team’s gel-like “optoDroplets” could provide “unprecedented access to manipulating and understanding the chemistry that allows membraneless organelles to function.” The research team believes that optoDroplets can also provide more insight into the pathology of intracellular protein aggregates linked to diseases such as Huntington’s, Alzheimer’s and amyotrophic lateral sclerosis (ALS), or “Lou Gehrig’s disease”.
Optogenetic control of intracellular proteins
Previously, most research on the proteins that compose membraneless bodies within cells has been conducted in vitro. Now, the Princeton team says it’s developed a method using a blue laser and optogenetics for viewing the proteins and their phase transitions in vivo.
In that method, the researchers inject modified light-sensitive proteins into living mouse and human cell samples. When cells containing the modified proteins are exposed to blue light from a laser, the protein condense into gel-like droplets that mimic membraneless organelles. Yet as soon as the blue light is turned off, the phase-shift reverses. Since the cells are nearly transparent, the phase changes can be watched and recorded in real time.
Protein aggregates and disease
Brangwynne and his colleagues also noted that when the cells were exposed to very intense blue light, or when the cells contained a high concentration of the optogenetically modified protein, solid protein aggregates would form and would not dissolve when the blue laser was switched off. According to the researchers, these lumpy, irreversible aggregates could be analogous to the intracellular protein clumps implicated in diseases like Huntington’s, Alzheimer’s and ALS.
In the paper, the team describes protein assembly experiments using FUS—an important intracellular protein consisting of ribonucleic acid (RNA) and an RNA-binding protein that helps repair damaged DNA. However, FUS proteins with genetic mutations can become “sticky,” creating the protein aggregates seen in nerve cells of ALS patients. The researchers found that the gel state of FUS, not the liquid state, promotes these irreversible protein aggregates in the cell. Surprisingly, these results contradict what previous in vitro studies have reported.
The researchers plan to further investigate other intracellular protein phase transitions and aggregate formation using optoDroplets.