The new probe’s sample end measures a mere 8 mm in diameter. [Image: Jürgen Popp, Leibniz Institute of Photonic Technology Jena and Institute of Physical Chemistry, Friedrich-Schiller University Jena]
Using state-of-the-art multi-core fiber and tiny gradient-index (GRIN) optics, a German team has packed three nonlinear-optical-imaging techniques into a single endoscope with a business end only 8 mm in diameter (Optica, doi: 10.1364/OPTICA.4.000496). The researchers believe that the new multimodal device—which combines coherent anti-Stokes Raman scattering (CARS), second-harmonic generation (SHG), and two-photon excited auto-fluorescence (TPEF)—has particular promise for replacing time-consuming biopsies for cancer, especially in real-time, surgical settings.
Beyond slides and staining
Conventional biopsies, the gold standard for distinguishing cancerous cells from normal ones, involve removing part of the suspect tissue and sending it to the lab, where it’s stained and put under the microscope for examination by a trained pathologist. The whole process can take days, and that particularly complicates the surgeon’s task of distinguishing between healthy and malignant tissue during an operation.
In recent years, multimodal nonlinear microscopy—specifically, approaches that combine CARS, SHG and TPEF—has emerged as a powerful potential alternative to biopsies, for doing histology on cancer and on other conditions such as inflammatory bowel disease in near real time. Bringing several modes of nonlinear optics to bear on a slice of tissue eliminates the need for labeling or staining, and can provide a diffraction-limited view with the right equipment.
And there’s the rub. Multimodal imaging techniques commonly involve bulky, tabletop-scale elements—and putting them together in a straightforward, handheld instrument suitable for surgical uses has posed a significant technical challenge.
The big stumbling block is the CARS component. Unlike SHG and TPEF, which require only a single excitation laser, CARS requires two overlapping, high-intensity beams of different wavelengths. The need for multiple beams automatically increases the CARS footprint, and the required scanning of the beams involves mechanisms tough to fit into the end of a fiber endoscope. Further, the high pulse intensities can lead to nonresonant background noise, via nonlinear interactions with the fiber channel itself, that can swamp the delicate CARS signal.
Multi-core fiber and GRIN elements
To get multimode imaging into a handheld fiber tool, the German research team, led by OSA member Jürgen Popp of the Leibniz Institute of Photonic Technology in Jena, looked first at the fiber part of the equation. Their setup relies in particular on a cutting-edge multicore fiber (from Fujikura Ltd., Japan) a half a millimeter in diameter, containing a whopping 10,000 light-guiding cores. This material—used for the imaging fiber that delivers the laser signal—preserves the spatial relationship between the beams at the entrance and output ends of the fiber. Hence the laser-scanning procedure can take place at the “lab” side of the fiber rather than the sample side, eliminating the need to pack bulky, power-consuming optics into the business end of the fiber endoscope.
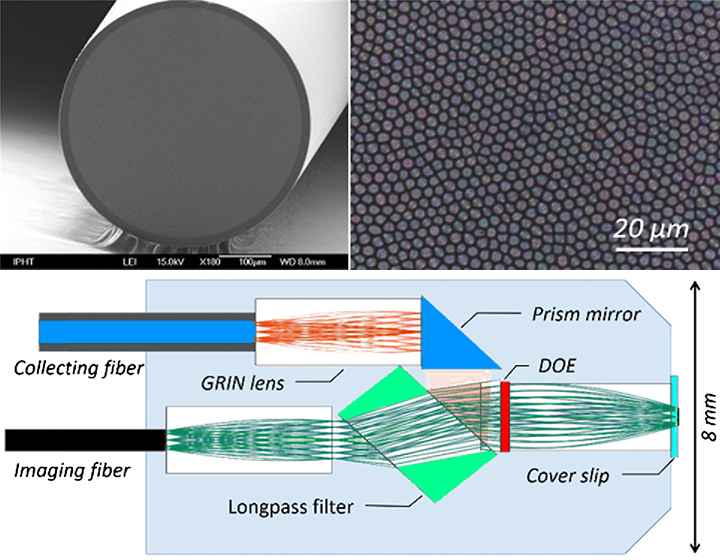
(Top) The new device uses a multi-core imaging fiber that packs 10,000 light guides into a 0.5-mm-diameter channel. (Bottom) The probe design combines tiny GRIN lenses to collimate and direct the beam and a longpass filter that directs nonlinear noise from the fiber to a separate “beam dump.” [Images: A. Lukic et al., Optica, DOI: 10.1364/OPTICA.4.000496]
When the laser light emerges from the imaging fiber, it passes through a GRIN lens only around 1.8 mm in diameter (designed by the firm Grintech Gmbh), which collimates the beam, and then through a longpass filter; the filter allows the excitation laser to pass through but shunts the nonresonant background noise to a “beam dump” elsewhere in the probe. A second GRIN lens trains the beam on the sample, and the nonlinear signal from the sample passes back through several GRIN optics into a separate collecting fiber for imaging at the other end.
Facilitating quick decisions
The Jena team successfully demonstrated the endoscope by scanning it across human skin samples, and acquiring multimode images—at a 2048×2048-px resolution, for a 300×300-µm sample size—that compared well with images obtained by much larger equipment. While the images showed some issues with “vignetting” at the edges, the team believes those can be overcome by tweaking aspects of the GRIN-element geometry and refractive-index profile.
Meanwhile, Popp and the team are upbeat about the tool’s potential in the clinic and, in particular, the surgical suite. “We hope that, one day, multimodal endoscopic imaging techniques could help doctors make quick decisions during surgery,” Popp noted in a press release, “without the need for taking biopsies, using staining treatments or performing complex histopathological procedures.”
