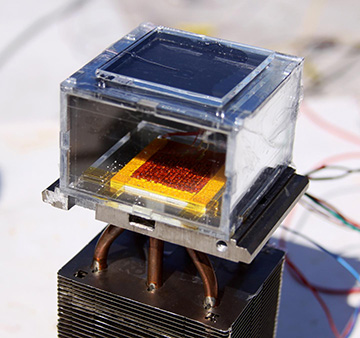
A water harvester built by a team from U.C. Berkeley and MIT combines metal-organic frameworks with solar energy, to pull liters of H20 from ambient, dry air. [Image: MIT photo, from laboratory of Evelyn Wang]
Before he became a Jedi Knight, Luke Skywalker worked with his uncle as a “moisture farmer,” in the business of harvesting water from the arid atmosphere of his home planet Tatooine. A team of scientists has now developed something that would have considerably simplified the lives of Luke and his Uncle Owen: a passive device that, with no more than the average energy input of a sunny day, can pull 2.8 liters of water from the air at relative humidity levels as low as 20 percent—a dryness common in desert areas (Science, doi: 10.1126/science.aam8743).
A MOF key to unlock atmospheric water
Getting water to the right place at the right time remains a problem for two-thirds of the world’s population. Yet, by some estimates, as much as 13,000 trillion liters of water worldwide are locked up in the air as water vapor. The research team, hailing from the University of California, Berkeley, and the Massachusetts Institute of Technology (MIT), USA, sought to unlock that vapor. And the key they used to do so lay in the intriguing family of materials known as metal-organic frameworks (MOFs).
MOFs, which have been on the scene for more than two decades, are cagelike arrays of multimetallic units tied together with organic-chain linkers. Because of their cagelike structure, MOFs have attracted considerable attention for applications such as storing methane gas in lightweight fuel tanks, carbon-dioxide capture, and adsorbing chemicals for catalysis.
Several years ago, the research team of the Berkeley-based, Jordanian-American chemist Omar Yaghi—who’s credited with the invention of MOFs, and who is a coauthor on the new paper—found that one MOF in particular, involving a combination of zirconium and adipic acid, was very good at binding to water vapor. That MOF, he reasoned, could thus form the foundation of a water-collecting system—if an energy-efficient way could be found to actually free the adsorbed water from its MOF cage.
Enter sunlight
The researchers found such a mechanism in the most abundant energy source of all: solar photons. Yaghi’s group joined forces with the mechanical-engineering team of MIT’s Evelyn Wang, the new paper’s other corresponding author, to develop a prototype device that could use solar energy to harvest the moisture grabbed from the air by the MOFs.
The prototype rests on intricate calculations to optimize the efficiency of the MOF structure for capturing moisture and for heat transfer from sunlight. But the design of the device, which operates completely passively, is simplicity itself. A layer of around 1 kg of dust-sized MOF crystals is compressed between a solar absorber (with an absorptance of 0.91) and a condenser plate at the ambient temperature. The MOF particles capture water vapor from the air, and the thermal energy from the sun drives the water out of the MOF cage and onto the condenser, from which it drips into a container.
In controlled experiments with the prototype, using a sunlight simulator, the team found that the 1-kg MOF chunk could extract as much as 2.8 liters of water in a day, and liberate it using solar energy, with no additional energy input. And the researchers believe that improvements, such as tweaking the structure and composition of the MOFs and the improving the efficiency of the solar-driven collector, could potentially double those output rates. “There’s a lot of potential for scaling up the amount of water being collected,” Yaghi noted in a press release. “It is just a matter of further engineering now.”
