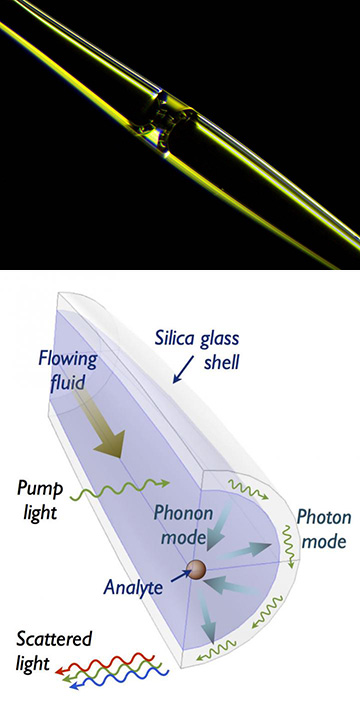
[Top] Photograph of a fluid meniscus inside the Illinois team’s opto-mechano-fluidic resonator (OMFR). [Bottom] In the OMFR, pump light circulating in a whispering-gallery resonance (WGR) mode in the fiber shell couples with the vibrational phonon mode in the fluid; changes in the phonon mode attributable to the passing particle, which are related to the particle’s mechanical properties, are read out in spectral changes in the coupled WGR light. [Images: University of Illinois]
A U.S. research team has devised a method for optical sensing of the mechanical properties of freely flowing micro/nanoparticle and bioparticle streams—at rates potentially topping 10,000 particles per second (Optica, doi: 10.1364/OPTICA.3.000585). The scheme, the researchers suggest, could open up a new diagnostic window into some diseases.
Knowledge gap
High-speed optical detection techniques—such as flow cytometry, used to count and analyze streams of suspended cells by passing them in front of a laser beam—excel at measuring large populations of particles and their optical properties, at blazing speeds approaching 50,000 particles per second. But photons can’t directly measure the mechanical parameters of the particles, such as density, compressibility and stiffness.
Instead, such measurements lie in the domain of mechanical sensors whose much slower operation has discouraged routine measurement of the mechanical parameters of large cell populations. That’s unfortunate, according to Gaurav Bahl, an assistant professor of engineering at the University of Illinois at Urbana-Champaign, USA—because, for certain diseases such as cancer and anemia, the mechanical properties of diseased cells could provide useful diagnostic information. As a result, says Bahl, “we have a substantial knowledge gap.”
Coupling photons and phonons
To start closing that gap, Bahl, along with Illinois colleagues Kewen Han and JunHwan Kim, sought a detection approach that combined speeds on the order of flow cytometry with the discrimination of mechanical sensors. They found such a scheme in a new “optomechanofluidic” detector that works by optomechanically coupling photons from a pump laser, used for measurement readout, with the phonons (quanta of vibrational energy) created as individual microparticles pass at high speed through a fluid channel.
In the system, the microparticle-containing fluid is pumped through a thin, hollow silica glass capillary, between 40 and 60 μm in diameter, that acts as an optomechanofluidic resonator (OMFR). As the fluid flows through the OMFR, a 1550-nm pump laser, coupled to the OMFR through a tapered fiber waveguide, feeds light into the silica shell, creating a whispering-gallery resonance (WGR) mode. At the glass-fluid interface, the WGR mode couples with the dispersed, long-range vibrational phonon mode present within the fluid channel.
As particles pass through the channel, they mechanically perturb the phonon mode in the fluid. Those perturbations are picked up in the coupled optical WGM, and register as changes in the vibrational power spectrum of the phonon mode, which is read out by proxy in the spectrum of light scattered from the WGR mode in the silica shell (see diagram above). Because the vibrational phonon modes in the fluid couple with the mechanical properties of the solid particles, the phonon mode spectral changes allow a real-time measurement of those properties in the optical beam.
Mechanical analysis at flow cytometry speeds
In tests using baker’s yeast and two types of microbeads, the team found that the system was able to sense multiple mechanical parameters in freely flowing particles, as expected. The researchers calculated that the sensor’s throughput could exceed 10,000 events per second—enabling, in their words, “[heretofore] inaccessible mechanical analysis of flowing particles at speeds comparable to commercial flow cytometry.”
Moreover, because the phonon modes permeate the entire fluid volume, the scheme enables the sensor to potentially “cast a perfect net” and measure every particle present, addressing an additional shortcoming of previous sensors. Overall, the team believes that, by allowing high-throughput analysis of the mechanical properties of large cell populations, the research potentially creates a new axis of measurement for disease diagnostics and study.
