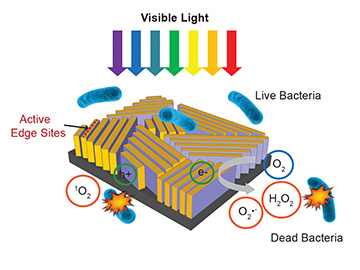
In the new device designed by Stanford and SLAC scientists, visible sunlight hitting thin flakes of molybdenum disulfide creates electron-hole pairs, which in turn trigger formation of reactive oxygen species that kill dissolved bacteria. [Image: C. Liu et al.]
In low-resource settings in the developing world, a common approach to disinfecting germy water is to pour it into a plastic jug, sit the jug in the sunlight, and wait—from 6 to 48 hours—for ultraviolet (UV) photons in the solar radiation to kill dissolved microbes. A team of scientists from Stanford University and the SLAC National Accelerator Laboratory, USA, has found a way to leverage considerably more of the solar spectrum and cut those wait times down to as little as 20 minutes (Nat. Nanotech, doi: 10.1038/nnano.2016.138).
Harnessing reactive oxygen species
The jug-in-the-sun method of purifying water counts on the direct action of high-energy UV photons to kill dissolved pathogens. But UV radiation accounts for only 4 percent of total solar energy, which makes the process glacially slow. (Another approach, simply boiling the water, uses precious fuel, and thus is not an option in many low-resource areas.)
One way to speed up solar water disinfection would be to use an alternative, indirect method that can tap a broader range of the solar spectrum. In such an indirect approach, solar photons don’t kill bacteria directly. Instead, they strike a semiconductor-based photocatalyst, creating electron-hole pairs. Those electrons and holes in turn react with water and dissolved oxygen to create reactive oxygen species (ROS) such as hydrogen peroxide and superoxide—potent chemical weapons that kill bacteria and pathogens by attacking essential macromolecules.
Such a method, in principle, could use the entire visible-light range of the spectrum, and dramatically speed up solar water disinfection. But there’s a catch: it’s been tough to find a semiconductor photocatalyst with the right band gap to do the job. One common photocatalyst, titanium dioxide, for example, has a band gap of 3.0 to 3.2 eV, well into the UV band and ineffective for harvesting visible-light photons. And pushing the band gap of conventional metal-oxide semiconductors into the visible range has been expensive and impractical.
MoS2 to the rescue
The Stanford-SLAC team, led by materials scientist Yi Cui, looked at another possible route: an emerging “2-D material,” molybdenum disulfide, or MoS2 (see “Optoelectronics in Flatland,” OPN, July/August 2015). As with other 2-D materials such as graphene, the properties of MoS2 in monolayer or few-layered sheets differ dramatically from those of the bulk material; in particular, the material shifts from having an indirect band gap of 1.3 eV in its bulk form to a direct band gap of 1.9 eV in monolayers. The team reasoned that, by tweaking the number and orientation of MoS2 layers, they could adjust the band gap to the sweet spot for grabbing visible light—and use the material as the foundation for a faster approach to solar disinfection.

Transmission electron micrograph of a patch of the photocatalytic surface, approximately 50 nm wide, showing the pattern of nanostructured MoS2 walls. [Image: C. Liu et al.]
The researchers began by creating molybdenum films using sputter deposition, and adding sulfur in a subsequent thermal step. The result was a 40-nm-thick film consisting of vertically aligned stacks of MoS2, each three to ten layers in thickness, in a wavy, hatched pattern. Testing revealed that the vertically aligned multilayered MoS2 sported a bandgap of approximately 1.55 eV—a level, in principle, that would allow the material to absorb the full range of visible light, up to 800 nm wavelength. That means that the potential to capture some 50 percent of solar energy, versus only 4 percent for the UV radiation that powers slow, direct solar disinfection.
Rapid disinfection
To test the concept, the team laid down the MoS2 film on a glassy carbon substrate around half the size of a postage stamp, and dropped it into a water sample containing E. coli bacteria. When the sample was placed in sunlight, the photocatalytic MoS2 device had inactivated more than 99.999% of the E. coli within two hours, well below the 6 to 48 hours required for direct solar disinfection. (After removal of the photocatalytic device, the reactive oxygen species quickly dissipate, leaving pure water behind.)
What’s more, the team found that by capping the vertical MoS2 strips with a 5-nm-thick layer of copper, the speed of disinfection could be increased still further—to a mere 20 minutes. The reason: the copper layer, in addition to being an ROS-generating catalyst in its own right, also creates a metal-semiconductor junction that enhances the electron-hole separation for the MoS2, supercharging its catalytic performance.
The new device has yet to be field-tested against contaminated water in the real world, and it obviously won’t solve all water problems—it can’t remove chemical pollutants, for example. But the research team believes the method does offer a relatively inexpensive way to speed up solar purification of microbe-tainted water in developing countries.
