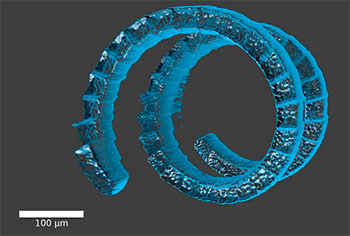
An animated multiphoton micromachined helix in a silk-protein hydrogel. Credit: Proc. Natl. Acad. Sci. USA, doi: 10.1073/pnas.1509405112
Current methods for shaping biomaterials, including soft- and photolithography, are limited to two dimensions and don’t offer much in the way of customization. Researchers at Tufts University report a new method inspired by 3-D printing that uses multiphoton absorption (MPA) of light to shape biomaterials that could be used in tissue engineering and biomedical implant applications (Proc. Natl. Acad. Sci. USA, doi: 10.1073/pnas.1509405112). The authors say that their multiphoton micromachining technique can generate high-resolution 3-D micro- and macroscale patterns deep within transparent silk-protein hydrogels.
The researchers, led by OSA Fellow Fiorenzo Omenetto, used low-energy (< nJ) femtosecond laser pulses to create 2-D and 3-D patterns in soft, transparent silk-protein hydrogels. They were able to achieve micromachining at a depth of 1 cm—reportedly more than 10 times deeper than any other biomaterial—at a lateral resolution of 5 µm. The researchers credit the impressive machining depth to the silk-protein hydrogel’s clarity. The “artificial vasculature” created by the micromachined tunnels allows cells to implant deep within the scaffold and gives the cells access to life-supporting air and nutrients. The researchers also report machining pores from 10 µm to 400 µm deep, which demonstrates the multiscale ability of their technique.
The authors report that their technique does not require toxic photoinitiator compounds that other 3-D printing techniques need, and that they can shape the silk hydrogels in a sealed, sterile environment, which is ideal for biomedical implants and tissue engineering. The researchers have observed similar results with multiphoton micromachining in in vitro experiments and preliminary in vivo experiments with mice.
